Diagnosis of Taenia solium infections based on “mail order” RNA-sequencing of single tapeworm egg isolates from stool samples
Henrik Sadlowski, Veronika Schmidt, Jonathan Hiss, Christian G. Schneider, Gideon Zulu, Alex Hachangu, Chummy S. Sikasunge, Kabemba E. Mwape, Andrea S. Winkler, Markus Schuelke
Tapeworm
species identification
taeniasis
stool microscopy
Taenia asiatica
Taenia solium
Taenia saginata
Neglected tropical diseases
SMART-seq 2
Single cell RNA sequencing
parasitology
Abstract
Here we present a detailed protocol for the identification of Taenia solium based on the few Taenia spp. eggs found in diagnostic stool samples. Our approach is based on "mail order" RNA sequencing of single eggs and can be performed in laboratories equipped with basic tools such as a microscope, a Bunsen burner, and access to an international post office for shipping samples to a next-generation sequencing facility.
This protocol describes sample collection and transport, isolation of individual Taenia spp. eggs, reliable disruption of individual Taenia eggs, and important considerations for shipping samples to a next-generation sequencing facility. We provide images and videos to help prepare the tools needed for the protocol. Additional information on our rationale for designing the critical steps can help implement the protocol in new environments.
Before start
Steps
Routine Diagnostic Workflow
Place 1 gram of a fresh native stool sample into a 15 mL Falcon tube and resuspend it in 10 mL PBS.
Centrifuge 2000x g
Remove the supernatant and all floating particles. Resuspend 1 volume of sediment in 2 volumes of PBS, mix well, and place a drop of the suspension on a microscopic specimen slide. Cover the sample with a cover slip. In some cases more PBS has to be added in order to reduce the density of the stool matrix.
Screen the sample on a microscope with 20x phase contrast lens for characteristic Taenia spp. or other helminth eggs.
If tapeworm eggs are detected, remove the coverslip and move the matrix with the PBS into an Eppendorf tube using a braod gauged pipette (e.g. cut the tip of a normal 200 μL laboratory pipette to increase the opening and to prevent blockage).
Clinical sample stability, transportation and storage
Store both, the Eppendorf tube with the matrix from the microscope slide and the stool sample at 4°C
Transport samples on cool packs (-20°C) in a styrofoam box to a laboratory that is connected to an international postal or courier service which accepts shipments on dry ice.
The maximum time investigated for storage under cool conditions (4°C in the refrigerator or in cold packs) was 8 days. For longer storage, samples can be frozen at -80°C.
Setup for egg preparation
Before proceeding with this protocol, arrange for shipment of the single cell sequencing reaction kits and/or components listed below on dry ice. We recommend that the following procedure be performed in a laboratory served by international mail or courier services to which the components are to be shipped. The remaining dry ice from these shipments can in turn be used to ship the samples to the sequencing facility.
Prepare the RNA stabilization buffer by adding the following components to an RNAse free tube:
- 10 μL dNTP mix containing 25 mM dATP, 25 mM dCTP, 25 mM dGTP, and 25 mM dTTP.
- 2.5 μL RNAse Inhibitor (40 U/μL)
- 1 μL 10% Triton X100
Add 0.6 μL of the RNA stabilization buffer to a clean 0.5 mL tube and immediately place it on ice.
Prepare two glass pipettes (see cartoon and movie below)
- Grasp a glass pipette (borosilicate) at both ends and place the middle part over a Bunsen or Butangas burner ( A ) until the glass begins to glow and become viscous ( B ).
- Pull the two ends slowly apart, causing the glass to taper at the glowing sections ( B ).
- Continue this movement until only a hair-thin glass thread connects the two ends ( C ).
- Remove the pipette from the Bunsen burner and, when cooled, breakt it at the tapered point ( D ).
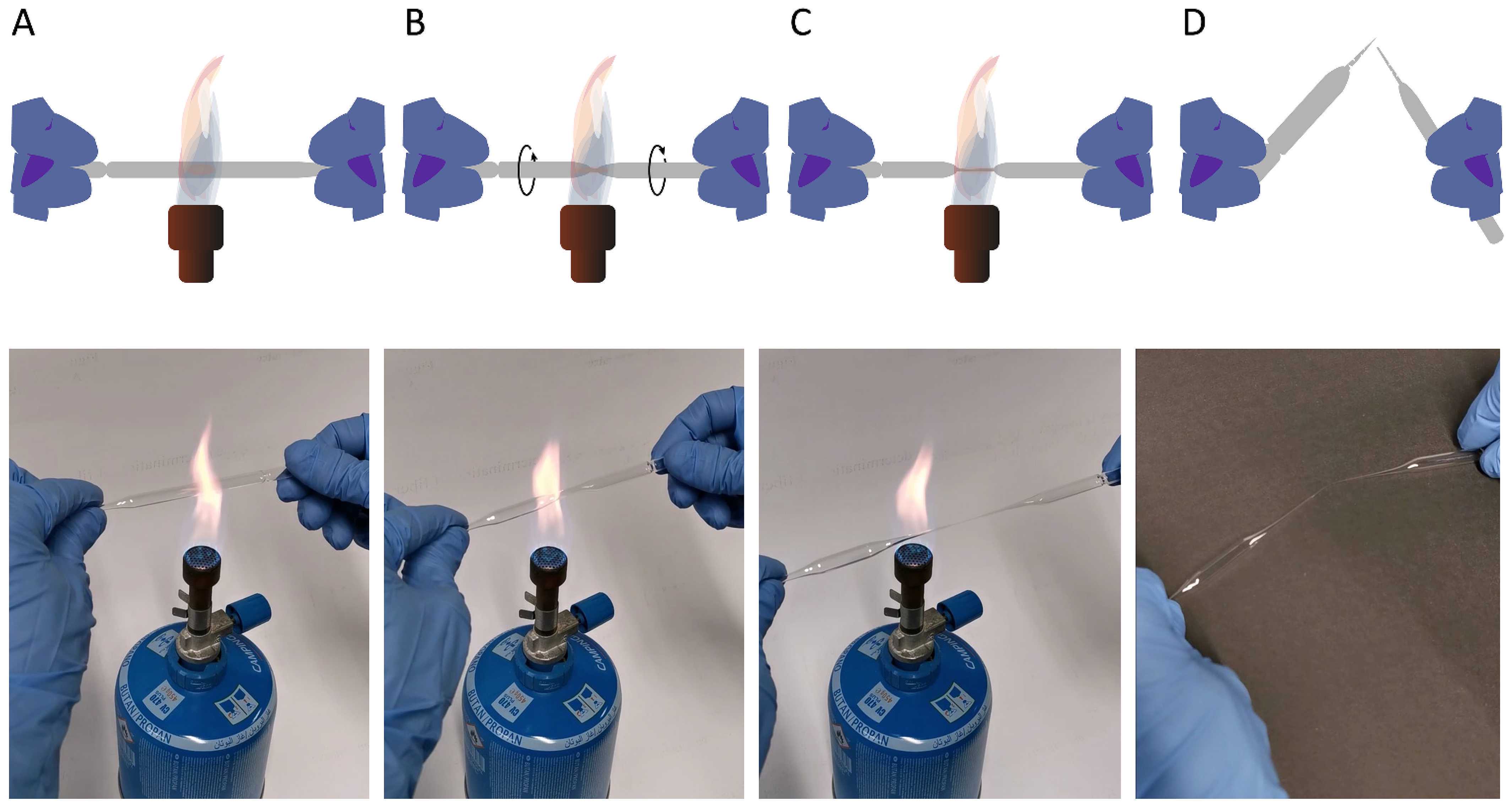
The movie can be watched from here: https://doi.org/10.6084/m9.f
Preparation of Taenia solium eggs for sequencing:
Resuspend approximately 50 mg of stool in 250 μL of PBS and apply 40 μL of the sample to a specimen slide. Do not cover the specimen with a coverslip. Focus an individual egg in the center of the field of view.
Aspirate 10 μL PBS using a 10 μL pipet tip and a 10 μL Eppendorf pipet. Insert the pipet tip into the stool sample and move it towards the egg in the center of the field of view under constant visual inspection through the ocular lens.
Aspirate the egg with its adhering debris with the pipette in a total volume of around 2 μL.
Release the aspirated eggs into 40 μL of fresh PBS on the microscopic slide. Exchange the pipet tip for a fresh one and aspirate the eggs again to move them into a fresh drop of PBS. These are the washing steps aiming to remove adherent organic material and bacteria.
Disruption of the eggs and stabilization of the RNA
- Aspirate two eggs in 4 μL PBS and place them on a fresh specimen slide. Insert the two glass pipettes into the drop of PBS ( A ) at 20x magnification and carefully place them at the left and right side of the egg ( B ). Press the needles carefully against each other to squeeze and ultimately destroy the egg under vision ( C ).
- Repeat this step with the second egg
- Aspirate the remaining PBS (usually around 3.5 μL due to evaporation) and add it to the SMART-seq buffer prepared in Step 10.
- Immediately place the tube on dry ice.
- Once the eggs are broken, they are not infectious anymore and can be shipped as "noninfectious" biological materials.

Shipment
Add a customs pro forma invoice declaring the value of the samples if they have been purchased or a nominal value of 1 USD if the samples are research samples with no monetary value. Add a letter stating that samples 4 μL are non-infectious lysates not containing any cells or dangerous substances.


