Using the HepaCometChip Assay for Broad-Spectrum DNA Damage Analysis
Norah A. Owiti, Norah A. Owiti, Simran Kaushal, Simran Kaushal, Lincoln Martin, Lincoln Martin, Jamie Sly, Jamie Sly, Carol D. Swartz, Carol D. Swartz, Jasmine Fowler, Jasmine Fowler, Joshua J. Corrigan, Joshua J. Corrigan, Les Recio, Les Recio, Bevin P. Engelward, Bevin P. Engelward
bulky lesions
comet assay
CometChip
DNA damage
genotoxicity
HepaCometChip
metabolic activation
single-strand breaks
Abstract
Exposure to DNA damaging agents can lead to mutations that cause cancer. The liver is particularly vulnerable because it contains high levels of Cytochrome P450 enzymes that can convert xenobiotics into DNA reactive metabolites that form potentially carcinogenic bulky DNA adducts. As such, current requirements for preclinical testing include in vivo testing for DNA damage in the liver, which often requires many animals. Given that efforts are underway in many countries to reduce or eliminate the use of animals in research, there is a critical need for fast and robust in vitro tests to discern whether xenobiotics or potential pharmaceutical agents can damage the hepatocyte genome. One possible approach is to leverage the alkaline comet assay, which is used to assess genotoxicity based on the ability of damaged DNA to become free to migrate toward the anode during electrophoresis. The comet assay, however, has several limitations. The assay is (i) slow and (ii) vulnerable to experimental noise, (iii) it is difficult to detect bulky DNA adducts since they do not directly affect DNA migration, and (iv) cell types typically used do not have robust metabolic capacity. To address some of these concerns, we have developed the “HepaCometChip” (a.k.a. the HepaRG CometChip), wherein metabolically competent cells are incorporated into a higher throughput CometChip platform. Repair trapping is used to increase sensitivity for bulky lesions: undetectable bulky lesions are converted into repair intermediates (specifically, single-strand breaks) that can be detected with the assay. Here, we describe a protocol for performing the HepaCometChip assay that includes handling and dosing of HepaRG cells and performing the CometChip assay. With its higher throughput, ability to capture metabolic activation, and sensitivity to bulky lesions, the HepaCometChip offers a potential alternative to the use of animals for genotoxicity testing. © 2022 The Authors. Current Protocols published by Wiley Periodicals LLC.
Basic Protocol 1 : HepaRG cell culturing and dosing
Basic Protocol 2 : CometChip assay
INTRODUCTION
DNA sequence information is grounded on the structure of the DNA bases, and so damage to the genome can lead to mutations that drive cancer (Friedberg et al., 2006; Hoeijmakers, 2001; Tubbs & Nussenzweig, 2017). Given the importance of DNA damage as a risk factor for cancer, there is a requirement that pharmaceutical agents be assessed for their genotoxic potential, and there is ongoing work by regulatory agencies to identify industrial chemicals that are potentially genotoxic. Importantly, some chemicals are relatively benign unless they are processed by Cytochrome P450 enzymes to form reactive metabolites in the liver. Therefore, analysis of DNA damage in hepatocytes is required to most effectively screen chemicals for their DNA damaging potential.
One way to measure DNA damage is via the comet assay, wherein DNA damage can be assessed based on the increased ability of damaged DNA to migrate when subjected to electrophoresis compared to undamaged DNA (Olive & Banáth, 2006; Ostling & Johanson, 1984; Singh, McCoy, Tice, & Schneider, 1988) (see below). Currently, the in vivo comet assay (wherein DNA damage in the liver is assessed) is a component of the required genetic toxicology test battery used by regulatory agencies in many countries. The in vivo comet assay involves treating rodents with chemicals, isolating cells from the livers of 40 to 50 rodents, and assessing those cells for the presence of DNA damage using the comet assay. While effective, there is a critical need to reduce reliance on animal genotoxicity testing. Indeed, mandates in the Frank R. Lautenberg Chemical Safety for the 21st Century Act, 2016, an amendment to the Toxic Substances Control Act (TSCA), require the Environmental Protection Agency to reduce and replace animal testing where scientifically reliable alternatives exist that would generate equivalent or better information. In 2013, the EU completed the ban on the sale of cosmetics for which there was animal testing. This applies to both cosmetic products and their ingredients, irrespective of whether there are alternatives. While animal testing is still allowed in the US, the NTP Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM) was created to propel the development of alternatives to animal use for chemical safety testing. In response to this pressing need, we have developed the HepaCometChip, a higher throughput comet assay that incorporates hepatocytes for detecting DNA damage, including damage caused by metabolically activated chemicals (see below) (Ngo et al., 2020).
The comet assay is a single-cell gel electrophoresis assay widely used for measuring DNA strand breaks. The comet assay is sensitive and versatile and has been used in more than 10,000 studies (Bajpayee, Kumar, & Dhawan, 2016; Cordelli, Bignami, & Pacchierotti, 2021; Olive & Banáth, 2006; Ostling & Johanson, 1984; Singh et al., 1988). The comet assay detects a broad range of DNA lesions based on the principle that damaged DNA is detectable in single cells by agarose gel electrophoresis (Azqueta & Collins, 2013). Specifically, while undamaged DNA is highly supercoiled and thus resistant to migration, DNA that harbors strand breaks can unwind, allowing DNA loops to migrate through an agarose matrix. When visualized using fluorescence microscopy, cells harboring DNA damage appear similar to a comet. A key step in the Comet assay is to incubate at high pH, which converts abasic sites and labile alkali sites into strand breaks. Single-strand breaks can also result from direct chemical reactions or enzymatic processing during DNA repair (Azqueta, Arbillaga, Lopez de Cerain, & Collins, 2013; Collins, Duthie, & Dobson, 1993; Muruzabal et al., 2020). Although base lesions do not affect DNA migration (and so cannot be directly detected), single-strand breaks are requisite DNA repair intermediates, so the presence of damaged bases can be deduced by their conversion into downstream excision repair intermediates.
The traditional comet assay involves placing cells in molten agarose onto glass slides (one for each condition), which is laborious, technically challenging, and noisy for experimentalists who have difficulty handling dozens of slides. The assay also requires that comets be imaged one by one while avoiding overlapping comets, and since 100 comets are analyzed per condition, this step is highly laborious. To help overcome these challenges, the CometChip was developed in the Engelward laboratory in collaboration with Bhatia (Ge et al., 2015; Ge et al., 2014; Weingeist et al., 2013; Wood, Weingeist, Bhatia, & Engelward, 2010). The underlying principle of the CometChip is that mammalian cells, including TK6, HepG2, and HepaRG, among many others, can be organized prior to electrophoresis in a compact agarose microarray wherein cells are arranged in a grid with ∼250 μm between microwells. This approach has numerous advantages: it avoids overlapping comets, the real estate requirement is small (hundreds of comets can be analyzed in a single well of a 96-well plate), there is a shared focal plane (such that only one or two images are needed for each sample), and automated image analysis software available. Also, since 96 samples can be analyzed in a single gel, sample-to-sample variation is suppressed. Of note, all the standard comet parameters can be ascertained (e.g., tail length, tail moment, etc.), though the most common approach is to measure the percentage of DNA in tails (OECD, 2016), which is recommended for the HepaCometChip (in part because each comet is self-calibrating). The features of the CometChip platform make it a relevant tool in the quest for a substitute for in vivo genotoxicity testing.
In addition to challenges with throughput and noise, additional shortcomings of the traditional comet assay include that (a) it is difficult to detect bulky lesions because their presence does not impact DNA migration, and (b) it is often the case that chemicals that form bulky lesions require metabolic activation. Although prior work has shown that bulky lesions can be gleaned from the presence of single-strand breaks created during nucleotide excision repair, the sensitivity of the assay is relatively low (Gedik, Ewen, & Collins, 1992; Hanasoge & Ljungman, 2007; Martin et al., 1999; Matsumoto et al., 2007). To address these limitations, we have increased the sensitivity of the assay for bulky lesion detection and created conditions amenable to metabolic activation. To increase the sensitivity of the CometChip, inhibitors of repair synthesis during nucleotide excision repair can be added to enable the conversion of undetectable bulky lesions into detectable single-strand breaks (Ngo et al., 2020). The ‘repair trapping’ approach was developed some time ago and has been used by several labs (Gedik et al., 1992; Hanasoge & Ljungman, 2007; Martin et al., 1999; Matsumoto et al., 2007). By incorporating HepaRG cells into the CometChip platform, we and others have shown that it is possible to detect bulky lesions that are formed as a consequence of P450 activation of xenobiotics (Barranger & Le Hégarat, 2022; Ngo et al., 2020; Seo, 2022; Seo et al., 2019).
HepaRG cells are being evaluated for use in drug development and toxicity testing programs to overcome a major deficiency of many current in vitro screening assays used in Tox21 and genetic toxicology testing (Andersson, Kanebratt, & Kenna, 2012; Buick et al., 2021; Franzosa et al., 2021; Seo et al., 2019). Xenobiotic metabolizing enzyme systems, critical determinants of drug toxicity, are absent in the cell lines used most often for regulatory genetic toxicology testing (e.g., human TK6 cells, MOLY, and CHO) and for the Tox21 and TOXCAST programs. HepaRG cells have been validated as competent in Phase I (e.g., CYP450 family) and Phase II (glucuronyltransferases, GSH-transferases, sulfotransferases) xenobiotic metabolism activities by multiple efforts, and they are a suitable replacement for cryopreserved primary human hepatocytes (the gold standard for chemical metabolism) (Bernasconi et al., 2019). As such, incorporation of HepaRG cells into the CometChip offers an excellent solution to the challenge of detecting DNA damage formed by chemicals that require metabolic activation.
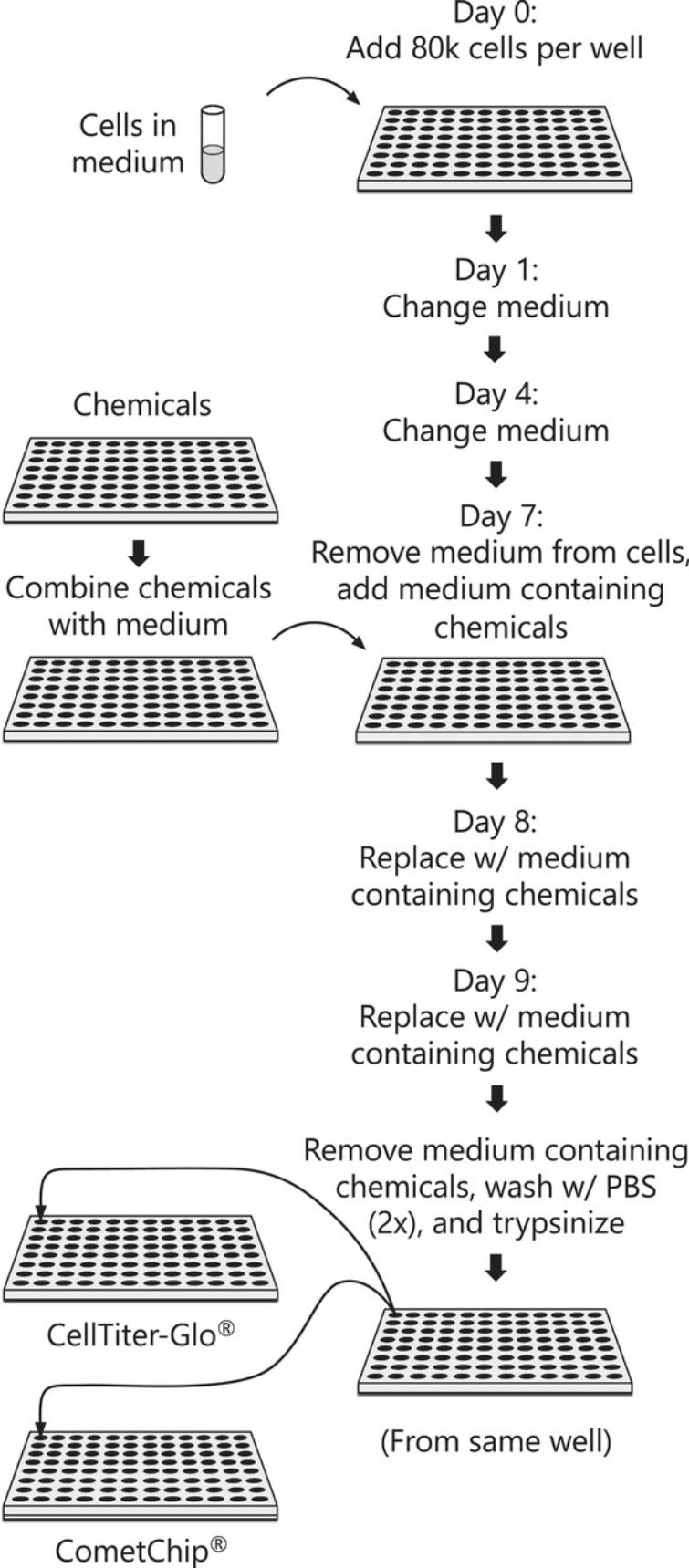
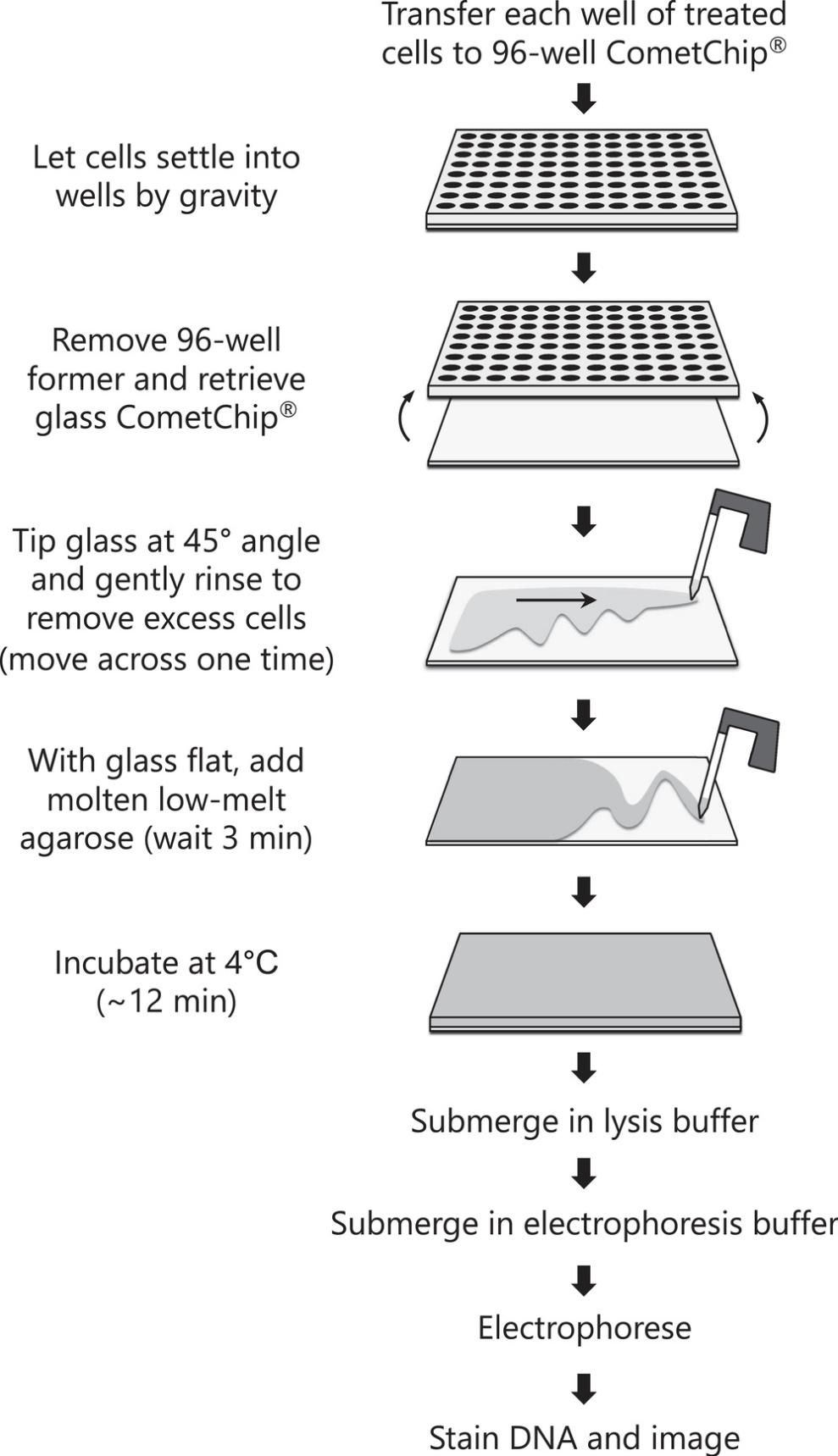
Here, we describe the protocol for performing the HepaCometChip assay using methods that are part of a formal protocol being developed as part of a validation effort under the NIEHS SBIR S2B entitled “CometChip: Development of a high throughput DNA damage assay in hepatocytes ” (2U44ES024698-04). We provide two basic protocols. Basic Protocol 1 details the procedure for handling and culturing HepaRG cells as well as dosing the cells with chemicals of interest (Fig. 1). Basic Protocol 1 also includes the option of incorporating repair inhibitors (hydroxyurea and cytosine arabinoside) to enable more sensitive detection of bulky adducts. Basic Protocol 2 then describes the CometChip assay protocol for chemically treated cells (Fig. 2) and acquiring images that can then be analyzed using any software for comet assays (though here we focus on the commercially available Trevigen comet analysis software). The approach provides an alternative to animal studies and a higher throughput genotoxicity test of industrial chemicals. Ultimately, detecting the DNA damaging potential of chemicals before people are exposed opens doors to cancer prevention.
STRATEGIC PLANNING
Handling HepaRG cells requires the specialized media and methods developed by Lonza. Before beginning, the user should have the HepaRG Thawing and Plating medium (1× Williams E Medium containing 1× GlutaMax and supplemented with HepaRG Thawing and Plating Media Supplement (Lonza, cat. no. MHTAP)) as well as the HepaRG Tox and Induction medium (1× Williams E Medium containing 1× GlutaMax and supplemented with HepaRG Pre-Induction and Tox Media Supplement (Lonza, cat. no. MHPIT)) prepared in advance. These media can be stored for up to 1 month at 4°C. Note that it takes many days to prepare cells for use in the HepaCometChip. Researchers should practice culturing HepaRG cells according to the manufacturer's instructions prior to performing the HepaCometChip assay.
If assessing bulky adducts and, therefore, incorporating hydroxyurea (HU) and cytosine-arabinoside (AraC) in the assay, it is paramount to first test the concentration of HU/AraC that induces <20% toxicity and with minimum baseline DNA damage in each cell type used. This is especially true when using metabolically competent cells other than HepaRG, as the concentrations recommended here are for HepaRG cells. This should be established prior to starting the experiments.
The hardware for the HepaCometChip is identical to the CometChip, and it is available from BioTechne. It is advisable to practice performing the CometChip with convenient cell types, such as TK6 or HepG2 cells, which are relatively easy to culture, prior to performing the assay with the HepaRG cells.
While straightforward, the rinse step of the CometChip assay after cell loading can be problematic. Briefly, after cells are loaded into the microwells, the excess cells are removed by shear force. Too much force causes cells to leave the microwells, while too little leads to excess cells outside of the microwells, which can complicate image analysis. It is advisable to practice this wash step, checking under the microscope for loading efficiency, before starting the HepaCometChip protocol. Note that the rinse step shown in Basic Protocol 2 Step 6 can be seen in Video 1.
Basic Protocol 1: HepaRG CELL CULTURING AND DOSING
This protocol describes the methodology for handling the HepaRG cells and performing a 3-day dosing regimen (to mimic the dosing schedule of in vivo genotoxicity assays, including the comet assay and the micronucleus assay) in the absence or presence of repair inhibitors. In brief, NoSpin HepaRG™ cells are obtained from the commercial source (Lonza, Research Triangle Park, NC) as fully differentiated hepatocytes in co-culture with biliary cells. The frozen cells are thawed and plated in William's E medium with Thawing and Plating supplement for 24 hr. On day 0, users will plate cells at 80,000 cells per well in collagen-coated 96-well culture plates (note that cell loading is effective above 10,000 cells per well). Cells are then incubated for 7 days after seeding, allowing the cells to regain peak metabolic function. On day 7, the cells are switched to a medium containing Pre-induction/Tox supplement containing either the test chemicals or the vehicle controls. Chemically treated media are refreshed on days 8 and 9, and 3 hr after the final treatment, cells are harvested for assessment of DNA damage by CometChip (with the option of using half the cells for cytotoxicity assays such as CellTiterGlo, which can be useful for dose selection; see below). See Figure 1 for an overview of the protocol.
Test chemicals should be formulated as concentrated stock solutions in DMSO or water. Treatment media containing chemicals should be prepared fresh each day of dosing. However, the concentrated stocks can be prepared once and frozen at −20οC until use unless stability data from the literature indicates that fresh daily preparation is needed.
Range finding experiments can be done using a cell viability assay such as CellTiterGlo, following the manufacturer's protocol. Generally, viability should not go below 50% since dying cells can elicit responses that damage DNA.
Materials
-
HepaRG Thawing and Plating Media Supplement (Lonza, cat. no. MHTAP)
-
70% ethanol
-
HepaRG Additive Pre-Induction and Tox Medium Supplement (Lonza, cat. no. MHPIT)
-
Hydroxyurea (HU) (Sigma, cat. no. H8627)
-
Cytosine arabinoside (AraC) (Sigma, cat. no. C1768)
-
Chemical(s) to be tested (e.g., Ethyl Methane Sulfonate (EMS) as positive control)
-
Solvent for dissolving chemicals (e.g., water and DMSO)
-
1× PBS (Lonza, cat. no. BE17-516F)
-
TrypLE Express Enzyme (ThermoFisher cat. no. 12605028)
-
No Spin HepaRG cell (Lonza, cat. no. NSHPRG)
-
Williams E Medium (no phenol red) (Gibco, cat. no. A12176-01)
-
Molecular biology grade water (ThermoFisher, cat. no. J70783.XCR)
-
GlutaMax 100× (Gibco cat. no. 35050-061)
-
37°C water bath
-
Laminar flow tissue culture hood
-
Conical tubes (15 and 50 ml)
-
Cell counter (e.g., Vi-CELL or using trypan blue)
-
96-well collagen-coated plates (ThermoFisher, cat. no. A1142803)
-
Mammalian cell incubators (37°C with 5% CO2)
-
Cell viability assay kit such as CellTiter-Glo (Promega, cat. no G9242) (optional)
-
96-well tissue culture plates
-
Chemical hood for handling stock solutions of chemicals to be tested
-
Plate shaker (optional)
-
Pipetman pipettes and tips
-
Multichannel Pipetman (optional but recommended) (P200 tips)
-
Sterile Reagent Reservoirs (Axygen, cat. no. RES-V-25-S)
-
Pipetaid (1 and 10-ml pipets)
-
Multichannel Aspirator (optional but recommended) (P200 tips)
-
0.2-μm sterile syringe filter (Pall Corporation, cat. no. 4433)
-
Syringes (1 and 5 ml)
Thawing HepaRG Cells (Day 0)
1.Pre-warm the HepaRG Thawing and Plating Medium in a 37°C water bath for ∼10-15 min.
2.In a biosafety hood, twist the cryovial cap a quarter turn to release the internal pressure and close it again.
3.Transfer the cryovial to the 37°C water bath. Hold the tip of the cryovial cap and gently agitate the vial for under 1 min until the frozen cells are almost thawed. Hold the tube to avoid letting the water in the water bath touch the rim of the cap.
4.Wipe the outside of the cryovial with 70% v/v ethanol using absorbent paper and place it in the hood.
5.Add 8.5 ml of pre-warmed HepaRG Thawing and Plating Medium into a conical tube and then transfer the cell suspension into the conical tube (for a total volume of 9 ml).
6.Rinse out the cryovial once with 1 ml of the Thawing and Plating Medium and add it to the conical tube (creates a total of 10 ml of cells).
Plating HepaRG Cells (Day 0)
7.Using a cell counter, determine the number of cells per ml.
8.Adjust cell density to 400,000 cells/ml using Thawing and Plating Medium, and plate ∼80,000 cells/well by transferring a volume of 200 μl of the cell stock solution into each well of a collagen-coated 96-well plate.
Optional : If measuring bulky adducts, prepare an extra plate for supplementation with HU and AraC, as described in steps 15-19.
9.Place plates in a mammalian cell incubator (37°C at 5% CO2).
Renew HepaRG Thawing and Plating Medium (Day 1)
10.Approximately 24 hr post-plating, pre-warm the HepaRG Thawing and Plating Medium in a 37°C water bath for ∼10-15 min before beginning the media renewal step.
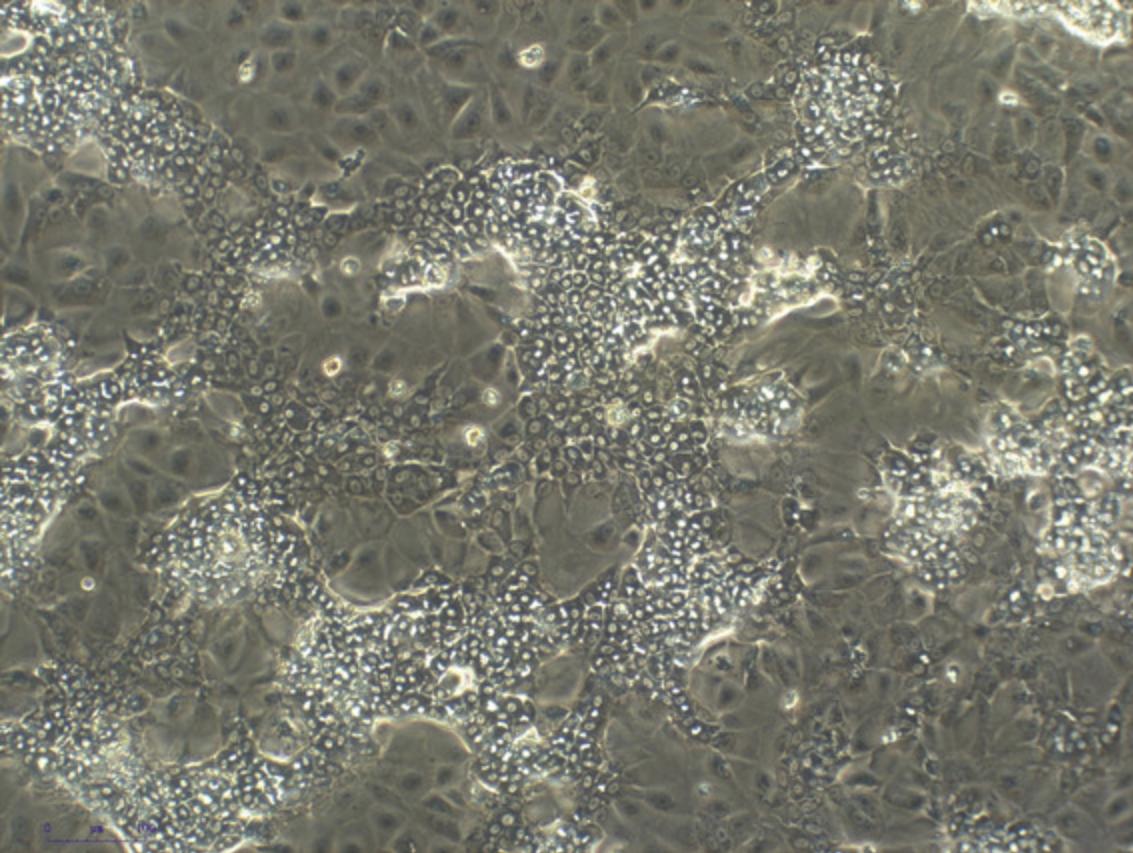
11.Take out the plates containing the HepaRG cells and inspect the cells under a bright field microscope to confirm adherence and morphology. Figure 3 shows the expected morphology.
12.Gently aspirate the HepaRG Thawing and Plating Medium from the plate(s) with a pipette and 200-μl tip or with a multi-channel aspirator, being careful not to touch the bottom of the well in order not to disturb the cells. Alternatively, invert the plate to dump culture media into an appropriate container, shaking gently once, to clear spent media from wells.
13.Add 200 μl of HepaRG Thawing and Plating Medium to each well. Place plate(s) back into the incubator for 3 days.
Renew HepaRG Thawing and Plating Medium (Day 4)
14.Repeat steps 10-13.
Exposure to Test Chemicals (Day 7)
15.Gently aspirate or pour off the medium from the plate(s), as described in step 12.
16.Add 200 μl HepaRG Tox Working Medium containing negative controls (such as DMSO or Media only), positive controls (such as ethyl methane sulfonate), and doses of chemicals of interest to generate dose-response curves at the end of the experiment.
Optional : If measuring bulky adducts, supplement the test media with 1 mM HU and 10 μM AraC.
17.Place plate(s) back into the incubator, recording the time.
Exposure with Test Chemicals (Day 8)
18.At 24 ± 1 hr post prior treatment, repeat the procedures described in steps 15-17.Record the time. Dispose of the chemical(s) according to your institution's chemical disposal policy.
Exposure with Test Chemicals (Day 9)
19.At 24 ± 1 hr post prior treatment, repeat the procedures described in step 18.
Removing Test Chemicals and Harvesting Cells (Day 9)
20.Pre-warm PBS and TrypLE for 15 min in a 37°C water bath before beginning step 21.
21.At 3 ± 1 hr after the final treatment, gently aspirate/pour off the medium from the plate. Dispose of the chemical(s) according to your institution's chemical disposal policy.
22.Rinse wells twice with 100 to 200 μl of pre-warmed PBS.
23.Remove PBS and detach cells by adding 100 μl/well prewarmed TrypLE and placing the plate in the incubator for 10 min. Following incubation, add 100 μl of pre-warmed HepaRG Pre-Induction and Tox Working Medium to each well to terminate trypsinization. Use a multichannel pipet to pipet up and down several times to create a single-cell suspension. Press the tips against the bottom of the plate to generate a shear force that will help to disaggregate the cells.
Basic Protocol 2: CometChip ASSAY
This protocol describes the CometChip assay, which follows the dosing of cells with chemicals of interest (Basic Protocol 1). HepaRG cells treated with chemicals and trypsinized with TrypLE to generate single cell suspensions are loaded into the CometChip, excess cells are washed away, and the Chip is capped using low melting point agarose. The cells are then lysed overnight in alkaline buffer. Following cell lysis, the DNA is unwound at high pH and electrophoresis is performed. The ‘comets’ are stained and imaged using a fluorescence microscope, and DNA damage is quantified as % tail in DNA. The methodology described here highlights all of these steps in detail. See Figure 2 for an overview of the CometChip protocol.
Materials
-
Triton X-100
-
Crystalline NaCl (VWR, cat. no. 7581)
-
Tris base (Sigma, cat. no. T1503)
-
Neutralization buffer (see recipe)
-
Electrophoresis Buffer (see recipe)
-
Cell Lysis buffer (see recipe)
-
SYBR gold (Invitrogen, cat. no. S11494)
-
1× PBS (Lonza, cat. no. BE17-516F)
-
HCl stock solution (1M)
-
96-well CometChip apparatus (R&D Systems, cat. no. 4260-096-CSK)
-
CometChip electrophoresis chamber (with power system) (R&D Systems, cat. no. 4260-096-ESK)
-
30-micron Microwell Chip (large glass slide with overlay of agarose harboring microwells) (R&D systems, cat. no. 4260-096-01)
-
96-well plate with treated HepaRG cells (Basic Protocol 1)
-
Mammalian cell incubators (37°C with 5% CO2)
-
Fluorescence microscope with 4× or 10× objective
-
Vacuum
-
Low melting point agarose
-
1 L flasks
1.To assemble the CometChip, gently remove the Chip (the glass plate coated with agarose harboring thousands of microwells) from the manufacturer's packaging. Pry open the CometChip apparatus using the CometChip key. Gently place the glass plate within the base of the CometChip apparatus. Gently replace the 96-well bottomless plate using the CometChip key to lower it onto the base.
2.Load 100 μl of treated cells (from Basic Protocol 1) into each well of a CometChip apparatus containing the CometChip (gel on glass).
3.Cover the CometChip apparatus using the lid provided and place it in a 37°C incubator for 15 min total to allow cells to settle into the microwells of the Chip. After 15 min, remove the Chip and gently rock back and forth to aid cell loading. Incubate for an additional 15 min in the 37°C incubator.
4.While the cells are loading, prepare ∼10 ml of 1% (v/w) low melting agarose and keep it at 37° to 42°C until ready for use. Alternatively, low-melting agarose can be prepared a day in advance and kept at 37° to 42°C.
5.Remove the Chip (gel on glass) from the CometChip apparatus by prying off the bottomless 96-well plate using the CometChip key provided.
6.Hold the Chip at a 45° angle over a dish (such as an empty p100 pipette box lid). Gently expel 5 ml warm PBS from a 5-ml pipet as you move the tip of the pipet from the top left to the top right corner perpendicular to the glass plate for a total of 5 s, as shown in Video 1.Remove the excess using a vacuum.
7.Visualize cell loading under a light field microscope to ensure the cells are sufficiently loaded.
8.Lay the Chip flat on a lab bench and add 7 ml of the molten 1.0% low melting point agarose (at 37° to 42°C) from step 4 onto the Chip to create an agarose overlay, using a 10-ml pipette. Gently release the agarose with a zig-zag pattern over the Chip from left to right, expelling the agarose between the wells. An alternative approach is to drip one drop of molten agarose onto the middle of each of the 96 wells. The end result should be an even layer across the surface. Allow the Chip to gelate at room temperature (3 min), followed by incubation at 4°C for ∼12 min.
9.While the low melting point agarose is solidifying, complete the process of preparing the cold lysis buffer by adding 1 ml Triton X-100 (complete lysis buffer is 1% (v/v) Triton X-100). Keep the lysis buffer at 4°C until use.
10.Submerge the entire glass Chip in cold lysis buffer and incubate overnight at 1° to 10°C (for example, place Chip in 100 ml lysis buffer in a p1000 pipette tip box lid or glass dish).
11.Place the Chip on the platform of a dry electrophoresis chamber (for example, using the Trevigen CometChip electrophoresis apparatus). Equilibrate the Chip by submerging it in an alkaline electrophoresis buffer in the electrophoresis chamber for 60 min at 1° to 10°C.
12.Using the same buffer, electrophorese the Chip in alkaline electrophoresis buffer for 50 min at 1° to 10°C with constant voltage (1 V/cm) and approximately 300 mA amperage.
13.Neutralize the Chip by submerging it in a neutralization buffer for 15 min at 1° to 10°C. Change the neutralization buffer and incubate for an additional 15 min.
14.Stain the Chip by submerging it in ∼30 to 50 ml 0.005× SYBR Gold diluted in 1× PBS for 60 min at 1° to 10°C in a pipette tip box lid protected from light.
15.Image immediately or return to neutralization buffer (0.4 M Tris, pH 7.4) until ready for imaging (ideally within 2 hr after staining).
16.Image using a fluorescent microscope at 4× (ideal) or 10×. At 4×, take one .tiff image for each 96-well to capture at least ∼100 comets per condition (two images may be necessary). To ensure the comet images are not saturated or overexposed, first image one well with multiple exposure times and choose the setting without image saturation. Then, use that setting to image all the remaining wells.
17.Analyze comets for percent tail DNA using a comet analysis software such as Trevigen Comet Analysis Software, following the manufacturers’ instructions. To analyze using the Trevigen software, open the analysis software to a 96-well plate format. Drag and drop the .tiff images into the respective plate wells and follow the wizard to analyze the images and export the results containing parameters such as % tail in DNA, tail length, and tail moment into an Excel file.
REAGENTS AND SOLUTIONS
Use deionized, distilled water in all recipes and protocol steps. Wear proper PPE when preparing NaOH, as the stock solution is highly corrosive.
Alkaline Unwinding and Electrophoresis buffer
Into a 1-L bottle, add 60 ml of 5 M NaOH solution (see recipe), 5 ml of 0.2 M Na2EDTA (see recipe), and 935 ml of distilled water. Adjust the pH to ∼13.5 (with NaOH or HCl). Store at room temperature for up to 2 weeks (Karbaschi et al., 2019).
Cell lysis solution
- 2.5 M NaCl (VWR, cat. no. 7581)
- 100 mM Na2EDTA (VWR cat. no. 4931)
- 10 mM Trizma base (base, Sigma, cat. no. T1503)
- 1% (v/v) TritonX-100
- NaOH pellets (VWR, cat. no. 7708-06)
- To prepare, dissolve 146 g crystalline NaCl, 37 g Na2EDTA, and 1.2 g crystalline tris in 900 ml distilled H2O and allow the contents to dissolve on a magnetic stirring plate. When fully dissolved, adjust the pH to 10 by adding NaOH pellets. Bring the volume to 1 L by adding distilled water. Adjust the pH to 10 accordingly (with NaOH or HCl). Store the solution at 4°C until ready to use, up to 8 weeks. Immediately before use, add TritonX-100 (Sigma cat. no. X-100) into the lysis buffer solution to a final concentration of 1% v/v. For example, add 1 ml TritonX-100 to 99 ml lysis buffer solution.
Cytosine arabinoside stock
Make 1000× stock of AraC by dissolving 11.6 mg AraC (Sigma, cat. no. C1768) in 5 ml distilled water. Vortex until fully dissolved and filter-sterilize through a 0.2-μm sterile needle filter fitted with a 5-ml sterile syringe. Make aliquots of the solution into 1.5 or 0.2-ml tubes and store at −20°C until ready to use.
Hydroxyurea (HU) stock
Make a 1000× stock of HU by dissolving 76 mg HU (Sigma, cat. no. H8627) in 1 ml distilled water. Vortex until fully dissolved and filter-sterilize through a 0.2-μm sterile syringe filter fitted with a 1-ml sterile syringe. Make aliquots of the solution in small 1.5 or 0.2-ml tubes and store at −20°C until ready to use.
Na2EDTA stock solution, 0.2 M
Transfer 75 g crystalline Na2EDTA (VWR cat. no. 4931) into a 1-L flask with a stirring magnet. Add 900 ml of distilled water and stir until completely dissolved. Bring the volume to 1 L. Store up to 1 year at room temperature.
NaOH stock solution, 5 M
Slowly add 200 g crystalline NaOH pellets (VWR, cat. no. 7708-06) to 900 ml distilled water while stirring on a magnetic plate until completely dissolved. Bring the total volume to 1 L. Do not use glassware to prepare or store NaOH. Prepare and store the stock solution with adequate ventilation and plastic containers. Store in a dedicated corrosives cabinet away from incompatible materials for up to 1 year.
Neutralization buffer
Take 400 ml of 1 M tris stock solution (158 g dissolved in 1 L of distilled water, pH 7.5) and bring the volume to 1 L by adding 600 ml of distilled water. Adjust the pH to 7.5 if necessary (with NaOH or HCl). Store at room temperature for up to 8 weeks.
COMMENTARY
Background information
The comet assay is broadly used to evaluate the levels of DNA damage in cells and tissues. In its original incarnation, the assay was used to detect double-strand breaks based on the principle that broken DNA migrates through a matrix more readily than undamaged DNA (which remains supercoiled under the conditions of the assay) (Ostling & Johanson, 1984). Soon thereafter, Singh et al. modified the assay to create the “alkaline comet assay,” wherein DNA exposed to high pH unwinds and reveals the presence of single-strand breaks (Singh et al., 1988). Despite being laborious and noisy, this version of the assay became highly popular because several DNA damaging agents introduce single-strand breaks, including radiation and oxidizing agents. Over time, the assay was modified in numerous ways to make it possible to detect a wide range of DNA lesions, including interstrand crosslinks and many types of base lesions. While effective for many applications, these methods do a relatively poor job of detecting bulky lesions, which on their own do not affect DNA migration and cannot be detected directly. Additionally, it is often the case that chemicals that create bulky lesions can do so because they are metabolically activated by P450 enzymes. We, therefore, set out to develop an assay that (a) allows for cells to convert undetectable bulky lesions into detectable strand breaks, (b) enables metabolic activation, and (c) leverages the 96-well CometChip platform. The combination of HepaRG cells with the CometChip is called the “HepaCometChip,” and the addition of chemical inhibitors of repair synthesis is an added feature. Results show that this is a robust approach for detecting the DNA-damaging potential of chemicals that require metabolic activation to form bulky DNA adducts (Ngo et al., 2020; Seo et al., 2019).
The development of the CometChip was an important design consideration. While the original comet assay is powerful, the procedure is onerous. Each sample requires a single glass slide, and hundreds of comets need to be identified and imaged individually to achieve 100 comets per condition (the standard number), rapidly becoming extremely time-consuming and tedious. Some progress was made by creating a glass slide to which 96 samples can be added (Trevigen, Inc.); however, this still requires tricky sample handling techniques and the same tedious imaging steps. To help overcome the problems of throughput and labor, the Engelward and Bhatia laboratories created the CometChip. Sample handling became far easier using a 96-well format and arraying mammalian cells on a grid. Additionally, the comets are on a shared focal plane, so hundreds of comets can be analyzed in a single image using an automated analysis program. Thanks to the increase in throughput and the vast reduction in time required for comet analysis, the CometChip is being increasingly adopted by researchers in academia, industry, and government. Compared to the traditional alkaline comet assay, the HepaCometChip offers sensitivity to bulky DNA adducts, ease of use, and higher throughput.
In terms of alternative approaches for detecting bulky lesions, the two most frequently used methods are mass spectrometry and unscheduled DNA synthesis. While highly sensitive, mass spectrometry is not often used due to the very high level of technical expertise required by the experimentalist and the high cost of analysis due to the need to have access to expensive equipment. Unscheduled DNA synthesis involves testing for incorporation of labeled nucleotides into repair patches created during nucleotide excision repair (which can be assessed in cells in G0/G1 or G2). This approach saves tremendously on costs but still requires particular technical expertise, so it is rarely adopted. Nevertheless, exciting advances are underway by the Niedernhofer laboratory, where they are creating a higher throughput flow cytometry-based unscheduled DNA synthesis assay. Once ready, this flow assay will provide a complementary approach for measuring the presence of bulky DNA lesions.
In terms of assay development, it is interesting to consider some of the design constraints. A key decision was which cell type to use for the HepaCometChip. HepG2 cells were first explored because they are easier to culture. However, the metabolic capacity of HepG2 cells is moderate. HepaRG cells are human cells that offer a much more robust and complete range of P450 expression. This is important because, for chemicals with unknown properties, one cannot know at the outset which P450 will be most important for metabolic activation. For this reason, HepaRG cells offer a great advantage. Another decision was whether to treat the HepaRG cells in a standard 96-well plate or load them into the CometChip and then expose them to test chemicals. An advantage of treating “on Chip” is that one can rapidly transition from treatment to lysis without intervening incubation steps. This may be helpful for some types of DNA damage that are repaired quickly and might be repaired during sample handling. Ultimately, however, it was deemed most advantageous to culture and treat the HepaRG cells in a 96-well plate since their behavior is best characterized under these conditions, and the cells might behave differently if cultured within agarose wells (which is essentially a low adherence condition).
While our previously published data on the HepaCometChip used a one-dosing regimen of 24 hr, here, we describe a 3-day regimen to mimic the dosing schedule of in vivo genotoxicity assays, including the comet assay and the micronucleus assay. The 3-day dosing regimen is used to assess the impact of preclinical candidates on human hepatocytes for regulatory applications.
While the HepaCometChip is useful for basic research, the main impetus for its development was an alternative to animal studies. Europe has banned the use of animals in chemical safety testing, and the NIH has initiated numerous programs that aim to support the development of in vitro alternatives to in vivo studies. The HepaCometChip offers a viable alternative to the in vivo comet assay. As a new alternative method, it is hoped that it will ultimately replace the in vivo comet assay as a way to identify potentially carcinogenic genotoxic chemicals.
Critical Parameters
While robust overall, key steps require optimization for successful use of the HepaCometChip. First, there is a need for careful loading into the CometChip. The first step of the loading process is straightforward: put cells in medium into the 96 wells and allow them to drop into the microwells by gravity. The next step, rinsing excess cells away, is more difficult. If too much shear force is applied (e.g., by having the pipet at the wrong angle or by extruding the PBS with too much force), cells will be bumped out of the wells, and there will be poor loading. If too little shear force is used (e.g., an insufficient volume of PBS), there will be many cells left on the surface of the gel rather than in the wells, which can lead to overlapping comets. As described above, it is highly recommended that the experimentalist practice this step prior to doing an actual experiment. This can be done by first examining the wells to make sure you can see them easily under the TC microscope, then loading, rinsing, and reexamining the wells to make sure most of the wells have cells and that there are not very many cells ‘off grid’. Once the experimentalist is proficient in this step, it is relatively easy to get excellent results from every experiment.
Two additional critical parameters are the quality of the cells and the quality of the chemicals used. Lonza products are highly reliable, but it is up to the experimentalist to ensure that cells are handled exactly according to the protocol. It is advisable also to read Lonza's HepaRG culturing instructions. Visual inspection is helpful in knowing that cells are properly differentiated. Also, a toxicity assay is helpful to know that cells are responding normally to the control chemicals. For this reason, it is recommended that a CellTiter-Glo assay (or similar toxicity test) be done in parallel with every experiment. In terms of the quality of the chemicals to be used, it is recommended that chemicals be purchased from a reliable source, ideally with quality control parameters tested (such as mass spectrometry). Chemical handling is also critically important because some chemicals degrade over time. For some chemicals, making small aliquots of frozen stock solutions can be effective.
In executing the experiment, almost all steps require standard pipetting skills. For new experimentalists, it is advisable to practice weighing water to ensure pipetting is perfectly accurate and that the pipettes are calibrated correctly. The only step that is unique to this protocol is the rinse step. As mentioned above, the rate at which PBS is expelled must be correct. As such, at a minimum, the experimentalist should practice expelling 5 ml of PBS while moving across the length of a 96-well plate. Ideally, the experimentalist will have an extra gel that can be used just for practicing the rinse step.
Troubleshooting
Please see Table 1 for a list of common problems with the protocols, their causes, and potential solutions.
| Problem | Possible reasons | Solution |
|---|---|---|
| Not enough cells loaded onto microwells when visualized at Basic Protocol 2 step 7 |
Excessive washing Too few cells in suspension due to incomplete detachment from plates. |
Washing too vigorously can cause cells to leave the microwells. The best way to avoid losing samples is to practice the loading step prior to starting the experiment (see Strategic Planning). Using a convenient cell type, load cells, rinse, and then look at the sample under the microscope to assess cell loading. Alternatively, dedicate a few 96 wells and a small amount of HepaRG cells to test loading before starting the experiment. Following the trypsinization process, thoroughly pipette cells up and down and check under the microscope to ensure that the cells are completely detached from the collagen-coated plates and in a single-cell suspension before proceeding with the assay. |
| Cells outside the microwells when visualized at Basic Protocol 2 step 7 | Not enough washing | Washing without enough force can lead to excess cells outside the wells, interfering with analysis. Practice cell loading before starting experiments (see Strategic Planning). Examine cells under the microscope and repeat the wash step if necessary. |
| High background DNA damage (long comet tails) in the untreated control samples visualized during imaging |
Improper cell handling Incorrect buffer pH Incorrect electrophoresis settings |
Cell stress can lead to an increase in baseline levels of DNA damage. Work quickly through the protocol and avoid letting the cells sit for too long at room temperature with no CO2 between media change and treatments. Only take out the cells when all the treatment solutions and media are warmed and ready to be added. Using the wrong pH can lead to inaccurate results. Always check the pH to ensure that the buffers are correctly made. Running the assay at higher than recommended voltage and/or for longer time than suggested can lead to significantly longer tails, leading to inconsistencies. Always double-check the electrophoresis settings before starting the process. |
| No increase in % tail DNA following HU/AraC addition (for positive control chemicals that leads to bulky lesions) | Incomplete repair trapping | Incomplete repair trapping can occur if the HU/AraC is not maintained throughout the treatment time. Always include a positive control, such as EMS, that does not require metabolic activation to ensure that the problem is due to incomplete repair trapping with HU/AraC and not other factors, such as incorrect buffers or lack of cell lysis. |
| High background (long comet tails) in the untreated control samples with HU and AraC visualized during imaging |
High concentrations of HU/AraC Using solvents that lead to high backgrounds with HU/AraC |
Addition of HU/AraC has been shown to lead to a slight increase in % tail DNA in untreated samples (Ngo et al., 2020). Using extremely high concentrations of HU/AraC can significantly increase this background and lead to a significant induction of cytotoxicity. It is therefore paramount to first test the concentration of HU/AraC that induces <20% toxicity and with minimum DNA damage baseline in each cell type used. For unknown reasons, some solvents such as DMSO can lead to an increase in background DNA damage in the presence of HU and AraC. It is important to keep the percent of DMSO used to less than 1% or switch to a different solvent whenever possible. |
Understanding Results
Visualizing HepaRG cells exposed to a
DNA damaging agent
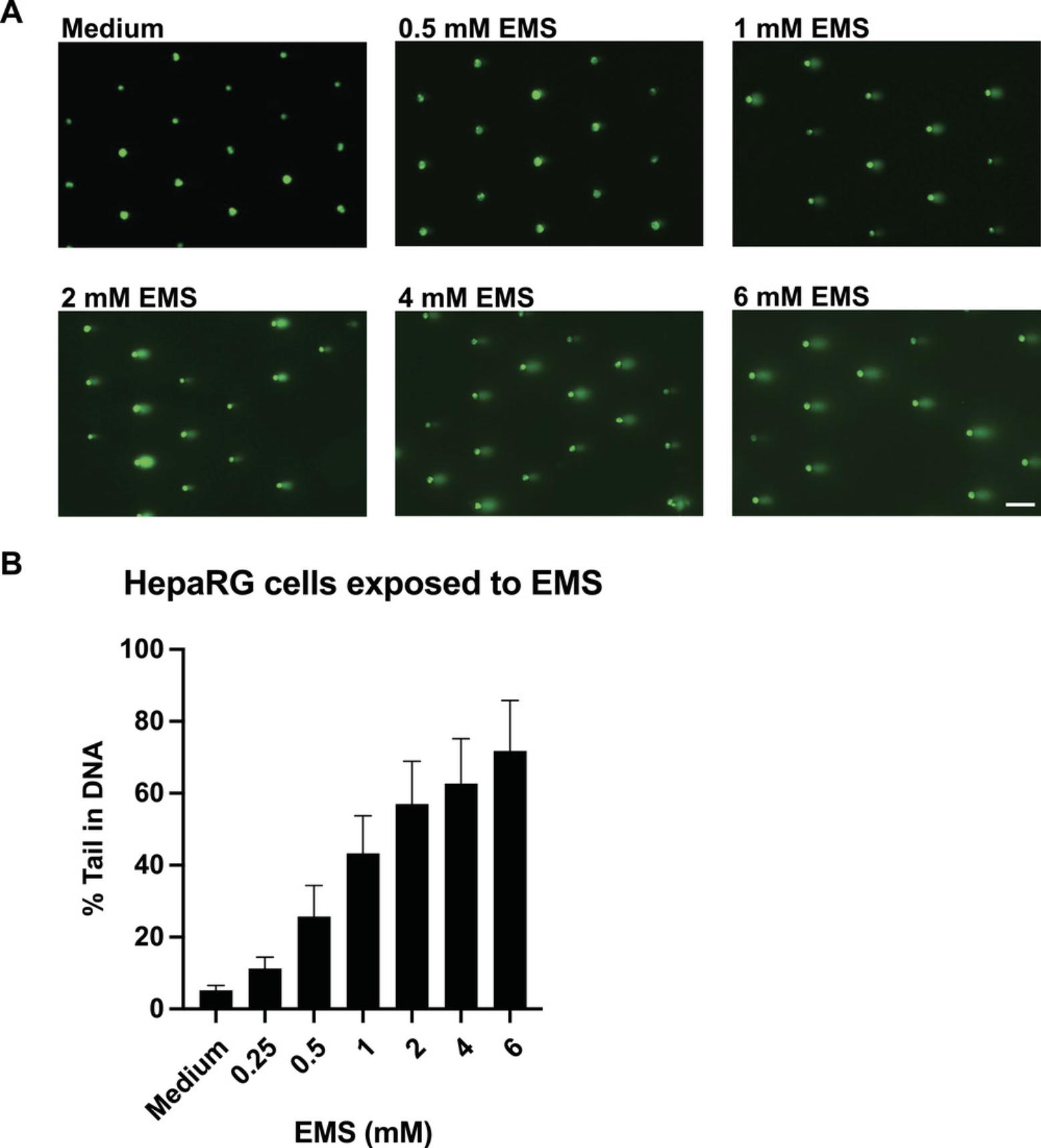
Efficiently loaded HepaRG cells should result in >70% loading in the microwells as observed under a microscope. Following exposure to positive control chemicals, comet-like shapes should be observed under a fluorescent microscope. Following analysis using comet analysis software, the % tail DNA should increase with an increase in the dose of chemical (for chemicals inducing damage). Figure 4A shows representative images of HepaRG cells exposed to DMSO or different doses of EMS (positive control). In the presence of EMS, there is a dose-dependent increase in % tail in DNA (Fig. 4B). In the case of HepaRG cells exposed to chemicals that induce bulky adducts (such as benzo[a]pyrene), a strong dose response is not observed in the absence of HU and AraC. However, following the addition of HU and AraC, there is a significant dose-dependent induction of % tail in DNA (Fig. 5). In a case where a tested chemical does not lead to an increase in % tail in DNA in the absence or presence of HU and AraC, then the said chemical is concluded not to induce strand breaks or DNA bulky lesions.
![Details are in the caption following the image HepaCometChip sample data showing HepaRG cells treated with B[a]P in the presence of HU and AraC. (A) Representative comet images of HepaRG cells exposed to no benzo[a]pyrene (B[a]P) and 8 μM B[a]P in the presence and absence of HU and AraC. Scale bar = 100 μm. (B) Dose response to B[a]P in HepaRG cells in the presence and absence of HU and AraC. % tail in DNA represented as the mean of four experiments ± SD.](https://static.yanyin.tech/literature_test/cpz1563-fig-0005-m.jpg)
Statistical analysis
Standard statistical approaches can be used to analyze CometChip data. For a small number of comparisons, a Student's t -test is appropriate. For dose-response data, ANOVA is recommended. Although thousands of comets can be analyzed in a single experiment, and it is possible to perform statistics using those individual values, it is advisable to repeat the experiment at least once to generate biological replicates. The ideal approach is to do three independent experiments and then take the median % tail DNA per well and the average and standard deviation of the three experiments.
Time Considerations
Table 2 indicates the typical time it takes at different stages of the protocol described in this manuscript.
| Procedure | Time (hr:min) | Additional notes |
|---|---|---|
| Thawing HepaRG cells | 00:05 | |
| Counting and plating HepaRG cells | 00:10 | This will vary based on the cell counting method. Time indicated is for counting using an automatic cell counter such as a Vi-CELL. |
| Renewing HepaRG cells | 00:05 | The medium needs to be renewed daily. |
| Exposing HepaRG to test chemicals | 00:30 | Each day of exposure. |
| Removing test chemicals and harvesting cells to single cell solution | 00:20 | |
| Loading cells onto the CometChip | 00:30 | |
| Washing excess cells and adding low melting agarose | 00:20 | |
| Alkaline cell lysis | 18:00 (overnight) | This can vary based on when the lysis step is started; >4 hr recommended. |
| Alkaline unwinding | 1:00 | |
| Electrophoresis | 0:50 | |
| Neutralization | 0:30 | |
| SYBR gold staining | 1:00 | |
| Fluorescence imaging | 1:00 | This can vary based on the microscope used. An automatic scanning microscope can take as few as 10 min per Chip and microscopes with a small field of view and manual capture can take up to an hour, depending on the operator. |
| Comet analysis | 00:30 | Based on the Trevigen automated imaging analysis software. Can vary if using other software that does not have automatic analysis. |
Acknowledgments
This work is supported by funding from the National Institute of Environmental Health Sciences SBIR S2B 2U44ES024698 to Integrated Laboratory Systems, Inc. (ILS).
Author Contributions
Norah Owiti : Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Original draft writing, review, and editing Simran Kaushal : Methodology, Draft review and editing; Lincoln Martin : Methodology, Project administration, Draft review and editing; Jamie Sly : Conceptualization, Formal analysis, Methodology, Draft review and editing; Carol Swartz : Conceptualization, Draft review and editing; Jasmine Fowler : Methodology, Draft review and editing; Joshua Corrigan : Validation, Draft review and editing; Les Recio : Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Original draft writing, review, and editing; Bevin Engelward : Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Original draft writing, review, and editing.
Conflict of Interest
Bevin Engelward is a co-inventor on the patent for the CometChip.
Open Research
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author.
Literature Cited
- Andersson, T. B., Kanebratt, K. P., & Kenna, J. G. (2012). The HepaRG cell line: A unique in vitro tool for understanding drug metabolism and toxicology in human. Expert Opinion on Drug Metabolism & Toxicology, 8(7), 909–920. doi: 10.1517/17425255.2012.685159
- Azqueta, A., Arbillaga, L., Lopez de Cerain, A., & Collins, A. (2013). Enhancing the sensitivity of the comet assay as a genotoxicity test, by combining it with bacterial repair enzyme FPG. Mutagenesis , 28(3), 271–277. doi: 10.1093/mutage/get002
- Azqueta, A., & Collins, A. R. (2013). The essential comet assay: A comprehensive guide to measuring DNA damage and repair. Archives of Toxicology , 87(6), 949–968. doi: 10.1007/s00204-013-1070-0
- Bajpayee, M., Kumar, A., & Dhawan, A. (2016). Chapter 1. The comet assay: A versatile tool for assessing DNA damage. In D. Anderson & A. Dhawan (Eds.) The comet assay in toxicology (pp. 1–64). Cambridge, UK: Royal Society of Chemistry. doi: 10.1039/9781782622895-00001
- Barranger, A., & Le Hégarat, L. (2022). Towards better prediction of xenobiotic genotoxicity: CometChip technology coupled with a 3D model of HepaRG human liver cells. Archives of Toxicology , 96(7), 2087–2095. doi: 10.1007/s00204-022-03292-4
- Bernasconi, C., Pelkonen, O., Andersson, T. B., Strickland, J., Wilk-Zasadna, I., Asturiol, D., … Coecke, S. (2019). Validation of in vitro methods for human cytochrome P450 enzyme induction: Outcome of a multi-laboratory study. Toxicology in Vitro , 60, 212–228. doi: 10.1016/j.tiv.2019.05.019
- Buick, J. K., Williams, A., Meier, M. J., Swartz, C. D., Recio, L., Gagné, R., … Yauk, C. L. (2021). A modern genotoxicity testing paradigm: Integration of the high-throughput CometChip® and the TGx-DDI transcriptomic biomarker in human HepaRG™ cell cultures. Frontiers in Public Health , 9, 694834. doi: 10.3389/fpubh.2021.694834
- Collins, A. R., Duthie, S. J., & Dobson, V. L. (1993). Direct enzymic detection of endogenous oxidative base damage in human lymphocyte DNA. Carcinogenesis , 14(9), 1733–1735. doi: 10.1093/carcin/14.9.1733
- Cordelli, E., Bignami, M., & Pacchierotti, F. (2021). Comet assay: A versatile but complex tool in genotoxicity testing. Toxicology Research , 10(1), 68–78. doi: 10.1093/toxres/tfaa093
- Franzosa, J. A., Bonzo, J. A., Jack, J., Baker, N. C., Kothiya, P., Witek, R. P., … Wambaugh, J. F. (2021). High-throughput toxicogenomic screening of chemicals in the environment using metabolically competent hepatic cell cultures. NPJ Systems Biology and Applications , 7, Article no. 7. doi: 10.1038/s41540-020-00166-2
- Friedberg, E. C., Aguilera, A., Gellert, M., Hanawalt, P. C., Hays, J. B., Lehmann, A. R., … Wood, R. D. (2006). DNA repair: From molecular mechanism to human disease. DNA Repair , 5(8), 986–996. doi: 10.1016/j.dnarep.2006.05.005
- Ge, J., Chow, D. N., Fessler, J. L., Weingeist, D. M., Wood, D. K., & Engelward, B. P. (2015). Micropatterned comet assay enables high throughput and sensitive DNA damage quantification. Mutagenesis , 30(1), 11–19. doi: 10.1093/mutage/geu063
- Ge, J., Prasongtanakij, S., Wood, D. K., Weingeist, D. M., Fessler, J., Navasummrit, P., … Engelward, B. P. (2014). CometChip: A high-throughput 96-well platform for measuring DNA damage in microarrayed human cells. Journal of Visualized Experiments: JoVE , Oct 18(92), e50607. doi: 10.3791/50607
- Gedik, C. M., Ewen, S. W., & Collins, A. R. (1992). Single-cell gel electrophoresis applied to the analysis of UV-C damage and its repair in human cells. International Journal of Radiation Biology , 62(3), 313–320. doi: 10.1080/09553009214552161
- Hanasoge, S., & Ljungman, M. (2007). H2AX phosphorylation after UV irradiation is triggered by DNA repair intermediates and is mediated by the ATR kinase. Carcinogenesis , 28(11), 2298–2304. doi: 10.1093/carcin/bgm157
- Hoeijmakers, J. H. (2001). Genome maintenance mechanisms for preventing cancer. Nature , 411(6835), 366–374. doi: 10.1038/35077232
- Karbaschi, M., Ji, Y., Abdulwahed, A. M. S., Alohaly, A., Bedoya, J. F., Burke, S. L., Boulos, T. M., Tempest, H. G., & Cooke, M. S. (2019). Evaluation of the major steps in the conventional protocol for the alkaline comet assay. International Journal of Molecular Sciences , 20(23):6072. doi: 10.3390/ijms20236072
- Martin, F. L., Cole, K. J., Orme, M. H., Grover, P. L., Phillips, D. H., & Venitt, S. (1999). The DNA repair inhibitors hydroxyurea and cytosine arabinoside enhance the sensitivity of the alkaline single-cell gel electrophoresis ('comet') assay in metabolically-competent MCL-5 cells. Mutation Research , 445(1), 21–43. doi: 10.1016/s1383-5718(99)00116-3
- Matsumoto, M., Yaginuma, K., Igarashi, A., Imura, M., Hasegawa, M., Iwabuchi, K., … Matsunaga, T. (2007). Perturbed gap-filling synthesis in nucleotide excision repair causes histone H2AX phosphorylation in human quiescent cells. Journal of Cell Science , 120(Pt 6), 1104–1112. doi: 10.1242/jcs.03391
- Muruzabal, D., Sanz-Serrano, J., Sauvaigo, S., Gutzkow, K. B., Lopez de Cerain, A., Vettorazzi, A., & Azqueta, A. (2020). Novel approach for the detection of alkylated bases using the enzyme-modified comet assay. Toxicology Letters , 330, 108–117. doi: 10.1016/j.toxlet.2020.04.021
- Ngo, L. P., Owiti, N. A., Swartz, C., Winters, J., Su, Y., Ge, J., … Engelward, B. P. (2020). Sensitive CometChip assay for screening potentially carcinogenic DNA adducts by trapping DNA repair intermediates. Nucleic Acids Research , 48(3), e13. doi: 10.1093/nar/gkz1077
- OECD. (2016). Test No. 489: In Vivo Mammalian Alkaline Comet Assay.
- Olive, P. L., & Banáth, J. P. (2006). The comet assay: A method to measure DNA damage in individual cells. Nature Protocols , 1(1), 23–29. doi: 10.1038/nprot.2006.5
- Ostling, O., & Johanson, K. J. (1984). Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochemical and Biophysical Research Communications , 123(1), 291–298. doi: 10.1016/0006-291x(84)90411-x
- Seo, J.-E. (2022). Evaluation of an in vitro three-dimensional HepaRG spheroid model for genotoxicity testing using the high-throughput CometChip platform. Altex , Online ahead of print. doi: 10.14573/altex.2201121
- Seo, J. E., Tryndyak, V., Wu, Q., Dreval, K., Pogribny, I., Bryant, M., … Guo, X. (2019). Quantitative comparison of in vitro genotoxicity between metabolically competent HepaRG cells and HepG2 cells using the high-throughput high-content CometChip assay. Archives of Toxicology , 93(5), 1433–1448. doi: 10.1007/s00204-019-02406-9
- Singh, N. P., McCoy, M. T., Tice, R. R., & Schneider, E. L. (1988). A simple technique for quantitation of low levels of DNA damage in individual cells. Experimental Cell Research , 175(1), 184–191. doi: 10.1016/0014-4827(88)90265-0
- Tubbs, A., & Nussenzweig, A. (2017). Endogenous DNA damage as a source of genomic instability in cancer. Cell , 168(4), 644–656. doi: 10.1016/j.cell.2017.01.002
- Weingeist, D. M., Ge, J., Wood, D. K., Mutamba, J. T., Huang, Q., Rowland, E. A., … Engelward, B. P. (2013). Single-cell microarray enables high-throughput evaluation of DNA double-strand breaks and DNA repair inhibitors. Cell Cycle , 12(6), 907–915. doi: 10.4161/cc.23880
- Wood, D. K., Weingeist, D. M., Bhatia, S. N., & Engelward, B. P. (2010). Single cell trapping and DNA damage analysis using microwell arrays. Proceedings of the National Academy of Sciences of the United States of America , 107(22), 10008–10013. doi: 10.1073/pnas.1004056107
Citing Literature
Number of times cited according to CrossRef: 3
- Xiaoqing Guo, Hannah Xu, Ji-Eun Seo, Application of HepaRG cells for genotoxicity assessment: a review, Journal of Environmental Science and Health, Part C, 10.1080/26896583.2024.2331956, 42 , 3, (214-237), (2024).
- Aris A. Polyzos, Ana Cheong, Jung Hyun Yoo, Lana Blagec, Sneh M. Toprani, Zachary D. Nagel, Cynthia T. McMurray, Base excision repair and double strand break repair cooperate to modulate the formation of unrepaired double strand breaks in mouse brain, Nature Communications, 10.1038/s41467-024-51906-5, 15 , 1, (2024).
- Leslie Recio, Jasmine Fowler, Lincoln Martin, Carol Swartz, Genotoxicity assessment in HepaRG™ cells as a new approach methodology follow up to a positive response in the human TK6 cell micronucleus assay: Naphthalene case study, Environmental and Molecular Mutagenesis, 10.1002/em.22575, 64 , 8-9, (458-465), (2023).

