Urine Lipoarabinomannan as a Biomarker for Mycobacterium tuberculosis and non-tuberculous mycobacterial infections
Jordan Manzer, Ibrahim Aboellail, Jerry A Nick, Delphi Chatterjee, Anita G Amin
Lipoarabinomannan
Cystic Fibrosis
M. abscessus
Cystic Fibrosis
M. abscessus
M. avium complex
NTM LAM
TB Diagnostics
Abstract
Lipoarabinomannan (LAM) is a unique cell wall component containing a fatty acid region with a large mannan core attached to poly-arabinan chains, sometimes followed by mannose capping in Actinomyces species. The hydrophobic region made of tuberculostearic acid (TBSA) and two palmitic acid chains linked to a phosphoinositol, anchors LAM to the mycobacterial cell wall. As a major constituent of all mycobacteria, including Mycobacterium tuberculosis (M.tb) , LAM has been shown to be a useful diagnostic biomarker in the identification of MTB infection in HIV positive (HIV+) patients. The size of LAM makes it difficult to analyze on GC-MS when using complex matrices such as urine, therefore chemical processing and derivatization is required. Two methods have been developed to quantify urine-LAM amounts, one by measuring D-arabinose and the other TBSA. Both components are unique and not metabolized through basic pathways in mammalian cells and can therefore serve as LAM surrogates. Using either chemical assay, urine-LAM can be detected in low quantities with sensitive GC-MS, providing a diagnostic tool for clinical assessment of infection. These methods are robust and have been applied to a significant number of TB and non-tuberculous mycobacterial (NTM) urine samples. These protocols provide additional sensitivity beyond what is required for assays of MTB in HIV+ patients, allowing for identification of both M.tb and NTM infections in immunocompetent hosts.
Before start
1. Use only glass tubes with phenolic screw caps with PTFE lining for all chemical derivatization reactions. The glass tubes should be rinsed with sterile MilliQ water and then cooked in a 400oF Kiln oven to get rid of all impurities (to reduce background on GC-MS) before use.
- Only glass pipettes, glass pasteur pipets and glass capillaries must be used for dispensing the chemicals.
3. All drying steps should be done in Nitrogen evaporator and make sure the samples are completely dry with no visible mositure on the inner walls of the tubes before proceeding to next step.
- Controls are prepared using urine from a healthy volunteer (TB non endemic area) and are spiked with M. tb CDC-1551 LAM.
Steps
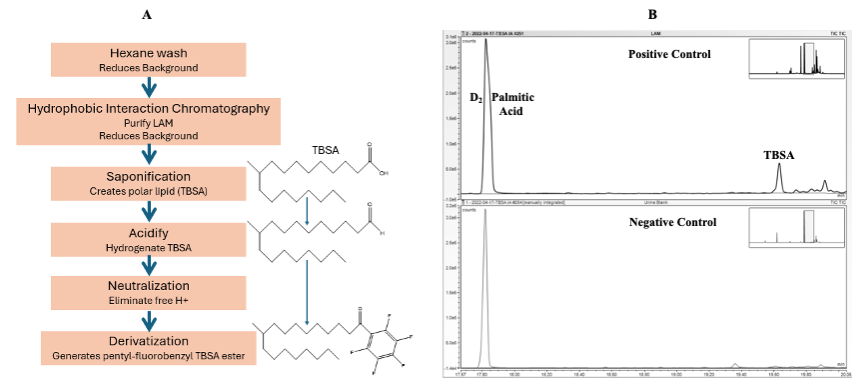
Urinary LAM Tuberculostearic acid Analysis
Hexane Extraction (Removes exogenous proteins and lipids from urine)
Using 1 mL of urine in a KIMAX 13 x 100mm glass tube with teflon screw cap, 1 mL of Hexane is added.
Vortex thoroughly for 20 seconds, two times.
Centrifuge at 3,500 rpm for 5 minutes.
1.3.1 If bubbly interphase exists, then repeat centrifugation for 5 to 10 minutes to achieve complete bilayer separation.
1.3.2 If the aqueous layer appears cloudy, or bubbly interphase persists repeat extraction with another 1 mL of Hexane.
Remove the upper Hexane layer and discard.
1.4.1 If there is precipitation at the bottom of the test tube, transfer the lower aqueous layer to a new tube, discard precipitate.
Dry the aqueous layer in a vacuum concentrator.
1.5.1 Hexane extraction is required only for urine samples obtained from pwCF for the NTM LAM analysis. For urine samples collected from TB suspects, Hexane extraction is not required, instead, 0.5 mL to 0.75mL of urine sample is transferred to a KIMAX 13 x 100mm tube and vacuum dried.
Hydrophobic Interaction Chromatography (Purify and concentrate LAM)
Clean 10 mL BIORAD Poly-Prep® column with two rinses of milli-Q water and one rinse of 200 proof ethanol, followed by additional two rinses with milli-Q water.
Transfer the final 0.1mL to the column using a pasteur pipet.
Add 2 mL of 5% n-propanol to the columns and allow to drip into reservoir.
Repeat with another 2 mL addition of 5% n-propanol.
Before the liquid completely drains out of the column add 2 mL of 15% n-propanol.
Repeat another 2 mL addition of 15% n-propanol.
Once the liquid completely drains, cap and cover the columns, remove the reservoir and replace with a fresh clean 13 x 100mm glass tube.
Add 2 mL of 40% n-propanol to the column and collect eluent in the glass tube.
Before the liquid drains add 2 mL of 65 % n-propanol.
Once both 40% and 65% n-propanol have been completely eluted into the glass tube, vortex to mix and dry in vacuum concentrator.
Add 2 mL of 5% n-propanol and 1 ml of room temperature Octyl SepharoseTM CL-4B to the clean column.
2.2.1 For elution from Octyl SepharoseTM CL-4B, a gradient of n-propanol (5%, 15%, 40% and 65%) in 0.1 M ammonium acetate is used.
2.2.2 Columns should never be allowed to dry out, add 5% or 15% n-propanol when needed to avoid dry columns before moving to elution steps (2.10 -2.14).
Allow the Octyl SepharoseTM CL-4B to settle in the column for 10 minutes and then pack the column by centrifugation for 10 minutes at 3,500 rpm.
Add 0.1 mL of 5% n-propanol to the vacuum dried aqueous layer (step 5) and centrifuge at 3,500 rpm for 30 seconds.
Remove the luer end fitting with snap off tip from the bottom of the columns and allow them to drip into a reservoir.
Before all the liquid completely drains add 2 mL of 5% n-propanol.
Repeat another 2 mL addition of 5% n-propanol before liquid drains.
Using a pasteur pipet, add the 0.1mL from the sample tube to the column.
Repeat with another 0.1mL 5% n-propanol addition to the sample tube and centrifuge at 3,500 rpm for 30 seconds.
Saponification, Acidification and Neutralization
Add 5 ng of Palmitic Acid 2,2-D2(Internal Standard) to each test tube.
3.1.1 Palmitic acid can be resuspended in Hexane and stored in -20oC.
Once completely dry add 0.15mL of ammonium hydroxide and gently vortex.
Dry under nitrogen flow in chemical fume hood.
Add 0.2mL of 0.25 N sodium hydroxide and incubate at 80oC for 1 hour.
Allow tubes to cool to room temperature and centrifuge 3,500 rpm for 30 seconds.
Add 0.5mL milli-Q water.
Titrate to a pH of 2 (pH paper) with dropwise addition of 3:1 (milli-Q water : sulfuric acid).
Add 0.5mL chloroform to the tube and vortex for 20 seconds 2 times.
Centrifuge at 3,500 rpm for 5 minutes.
Remove the upper, aqueous layer and discard.
Dry the lower chloroform layer under nitrogen flow.
Esterification
In a chemical fume hood, add 0.1mL of acetonitrile, 0.07mL ethyldi-isopropylamine, and 0.02mL 2,3,4,5,6 pentafluorobenzyl bromide.
Centrifuge at 3,500 rpm for 20 seconds to bring each solution to bottom of the test tube ensuring proper concentration.
Incubate at room temperature for 30 minutes.
Dry under nitrogen flow.
GC-MS analysis
Resuspend the dried sample in 0.1 mL of Hexane.
Using a pasteur pipet, transfer the sample to a GC vial.
Inject on GC-MS in negative chemical ionization with a low polarity J&W VF-5ms fused silica capillary GC column.
GC Oven settings: oven temperature was held at 50°C for 1 min and programmed at 20°C / min to 150°C and then programmed at 10°C / min to 310°C. (MS transfer line temperature: 315oC, Ion source temperature: 250oC).
Using Selective Ion Monitoring (SIM)
5.5.1 TBSA ester derivative anion at m/z 297.3 with a peak at 19.6-19.9 minutes retention time.
5.5.2 D2Palmitic acid internal standard anion at m/z 257.3 at 17.7-18.1 minutes retention time.

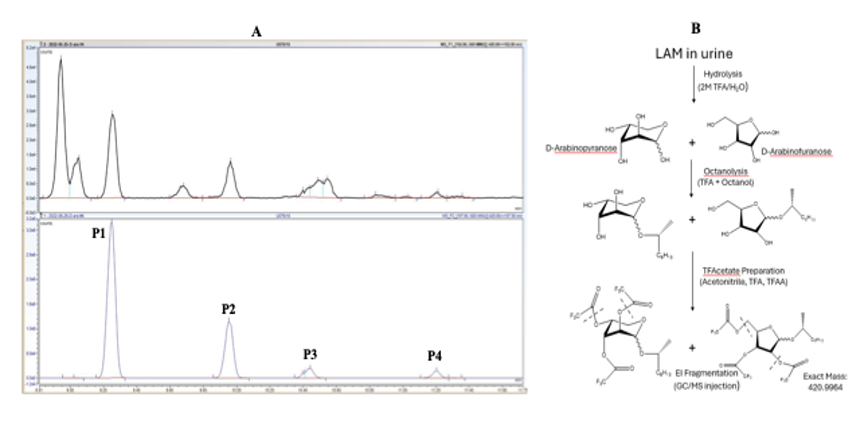
Urinary LAM D-Arabinose Analysis
Hexane Extraction (Removes exogenous proteins and lipids from urine)
Perform Hexane extraction same as shown in TBSA protocol, steps 1.1 - 1.5.
Hydrophobic Interaction Chromatography (Purify and concentrate LAM)
Purify and concentrate LAM using steps 2.1 - 2.18 (the same as stated for TBSA).
Hydrolysis
Add 200 ng of D-(UL-13C5)-Arabinose internal standard to each tube.
In chemical fume hood, add 0.2mL of freshly made 2 M Trifluoroacetic Acid (TFA).
9.2.1 2M TFA should be made fresh and stored on ice before use.
Screw Teflon® caps on tightly and incubate at 120oC for 2 hours.
Dry under nitrogen flow.
Octanolysis
In chemical fume hood, add 0.1 mL of (R)-(2) octanol and 0.02 mL concentrated TFA to each tube.
Incubate at 120oC overnight (16 – 18 hours )
Dry under nitrogen flow.
10.3.1 Make sure to dry the sample completely before proceeding to the next step. The drying takes longer than normal.
Acetylation
In a chemical fume hood, add 0.1 mL of chilled acetonitrile.
11.1.1 Acetonitrile must be chilled on ice prior to addition.
'
Add 0.01mL concentrated TFA.
Add 0.2 mL of trifluoroacetic anhydride.
Incubate at 55oC for 20 minutes.
Dry under nitrogen flow.
11.5.1 Acetylated samples can be stored at -20oC and are stable for about a week.
GC-MS analysis
Resuspend samples in 0.1 mL chloroform.
Using a pasteur pipet, transfer the sample to a GC vial.
Inject on GC-MS in electron ionization with Mid Polarity Phenomenex® ZB-5HT ZebronTM InfernoTM.
GC oven settings: oven temperature was held at 50°C for 1 min and programmed at 20°C/min to 150°C and then programmed at 2.5°C/min to 215°C. (MS transfer line temperature: 275oC, Ion source temperature: 300oC).
Using selective ion monitoring, look for 4 peaks between 10 minutes to 12 minutes retention time.
12.5.1 The ions m/z 420.9 (parent ion) to 192.9 (daughter ion), and m/z 425.9 (parent ion) to 197.9 (daughter ion) were monitored respectively for D-Ara and D-(UL-13C5)-Arabinose (internal standard).
Urinary LAM based on D-arabinose can be calculated using the equation below

Advantages
The quantification of of D-arabinose and TBSA is emerging as a valuable tool for the detection of non-tuberculous mycobacterial (NTM) infections, particularly in immunocompromised patients. This non-invasive test offers several advantages over traditional diagnostic methods, which often require respiratory samples and complex laboratory processing. Urine LAM testing may facilitate earlier diagnosis, and although it has been extensively utilized in diagnosing tuberculosis, its application for NTM infections is still being explored. These methods appear to have great promise in assessment of NTM infection in at-risk populations, especially when the disease burden is low such as in CF. To date these assays have been used successfully to identify pwCF with a history of both M. avium complex or M. abscessus infection, as well as a marker of treatment response of M. abscessus to phage. The use of these assays is currently being tested in a cohort of pwCF without a history of NTM positive cultures (ClinicalTrials.gov: NCT04579211), in order to determine the utility of urine LAM as a screening tool for NTM in this high risk population. These assays are also being validated in well-defined disease states using samples banked from the CFF-sponsored PREDICT Trial: Prospective Evaluation of NTM Disease in CF (NCT02073409) and the Prospective Algorithm for Treatment of NTM in Cystic Fibrosis Trial (PATIENCE: NCT02419989). The capacity of these assays to track response to treatment linked to novel therapeutics is being tested in the FDA-sponsored Phase 1b, Multi-center Study of IV Gallium Nitrate in Patients with Cystic Fibrosis who are Colonized with Nontuberculous Mycobacteria (ABATE Trial: NCT04294043) and the CFF-sponsored P r OS pec T ive ST andardized A ssessment of M ycobacterio P hage (POSTSTAMP) Trial (NCT06262282). Together, these propspective clinical trials will provide a clear assessment of the performance characteristics of this urine LAM assay in different stages of infection, disease and treatment response in the CF population. These results will likely be applicable to other forms of lung disease at-risk for NTM, as well as the potential to detect other presentations of NTM infection.

