Transfer of RNA from Agarose Gels onto Membranes
Jonathan Houseley, Cristina Cruz
Abstract
Over the past decade a plethora of noncoding RNAs (ncRNAs) have been identified, initiating an explosion in RNA research. Although RNA sequencing methods provide unsurpassed insights into ncRNA distribution and expression, detailed information on structure and processing are harder to extract from sequence data. In contrast, northern blotting methods provide uniquely detailed insights into complex RNA populations but are rarely employed outside specialist RNA research groups. Such techniques are generally considered difficult for nonspecialists, which is unfortunate as substantial technical advances in the past few decades have solved the major challenges. Here we present simple, reproducible and highly robust protocols for separating glyoxylated RNA on agarose gels and heat denatured RNA on polyacrylamide–urea gels using standard laboratory electrophoresis equipment. We also provide reliable transfer and hybridization protocols that do not require optimization for most applications. Together, these should allow any molecular biology lab to elucidate the structure and processing of ncRNAs of interest.
Introduction
Northern blotting methods allow for simultaneous quantification and molecular weight determination of RNA. Although superseded by qPCR and sequencing methods for routine mRNA quantification, northern blotting is the method of choice when complex mixtures of overlapping species are under investigation. This is particularly true when studying RNA processing by complexes such as the exosome, and generally aids in resolving the behavior of differentially expressed RNA isoforms. Historically, northern analysis has been something of a black art; running a high-quality formaldehyde gel required substantial skill and a little luck, while radioactive probing of RNA membranes often resulted in terrible cross-hybridization and invisible signals. Fortunately, technology has moved on such that modern northern analysis methods are simple and robust.
Electrophoretic separation of single-stranded RNA is more complex than double-stranded DNA as RNA forms strong secondary structures that impede separation by molecular weight in a gel matrix. For analysis of high molecular weight RNA in agarose gels, chemical modification of guanine is the preferred method to melt secondary structure, which disrupts C:G base pairing and allows single stranded RNA to migrate according to size. Although formaldehyde has been widely used for this purpose [1], it is not ideal due to issues with sample migration and batch-to-batch variation. Furthermore formaldehyde gels release toxic formaldehyde gas; this did not overly concern early investigators eager to replace the hideously toxic denaturant methyl mercury used in the first northern blotting protocols [2,3], but is clearly a problem in modern labs. To circumvent these issues glyoxal was long ago suggested as an effective RNA denaturant [4], but originally required technically awkward buffer recirculation. This problem was solved by the introduction of BPTE running buffer, which allows agarose gels of glyoxylated RNA to be run with no more difficulty than a normal DNA agarose gel [5].
High-resolution separation of small RNA fragments (~20–250 bp) is best performed on denaturing acrylamide gels, which rely on heat and urea rather than chemical modification to prevent secondary structure formation. The technique is identical to traditional sequencing gel electrophoresis [6], however, the apparatus used for sequencing is not practical for northern blotting and standard protein gel electrophoresis systems are well-suited for this purpose. Helpfully, many of the complications inherent to running a high quality sequencing gel can be safely ignored unless base-pair resolution is required.
Separated RNA is transferred to a membrane by capillary transfer for agarose gels or using an electroblotting system for acrylamide gels [7,8,9]. Various different membranes and transfer conditions have been described but we find charged nylon membrane best for all standard applications [10], and observe little difference between transfer methods. Similarly, many combinations of probes and hybridization buffers can be used to detect RNA species, each having their own strengths and weaknesses. Here we provide a protocol for using RNA probes transcribed from PCR products; in our hands these are both the most reliable and the most sensitive, and as such are the probe of choice for new users [11,12,13,14]. We also provide probing conditions for use of synthetic oligonucleotides, which are widely used in RNA processing analysis since they provide unparalleled resolution of intermediates, and for random-primed DNA probes.
The source of RNA used for northern blotting is rarely critical as long as it is of high quality ( see Note 1 ), and therefore in this chapter we focus purely on the gel systems and hybridization methods.
Before start
It is recommended to read through the guidelines before starting any work with RNA.
Steps
Transfer of RNA from Agarose Gels onto Membranes
Cut a 15 × 15 cm piece of nylon membrane, two 15 × 15 cm pieces of Whatman paper and a 15 × 46 cm piece of Whatman paper.
Cut a corner of the nylon membrane and label the membrane with pencil.
Soak the 15 × 15 cm Whatman paper and the membrane in 6× SSC in the same plastic box.
To set up the transfer apparatus (see Fig. 1), fill a box with 6× SSC up to about 2 cm deep.
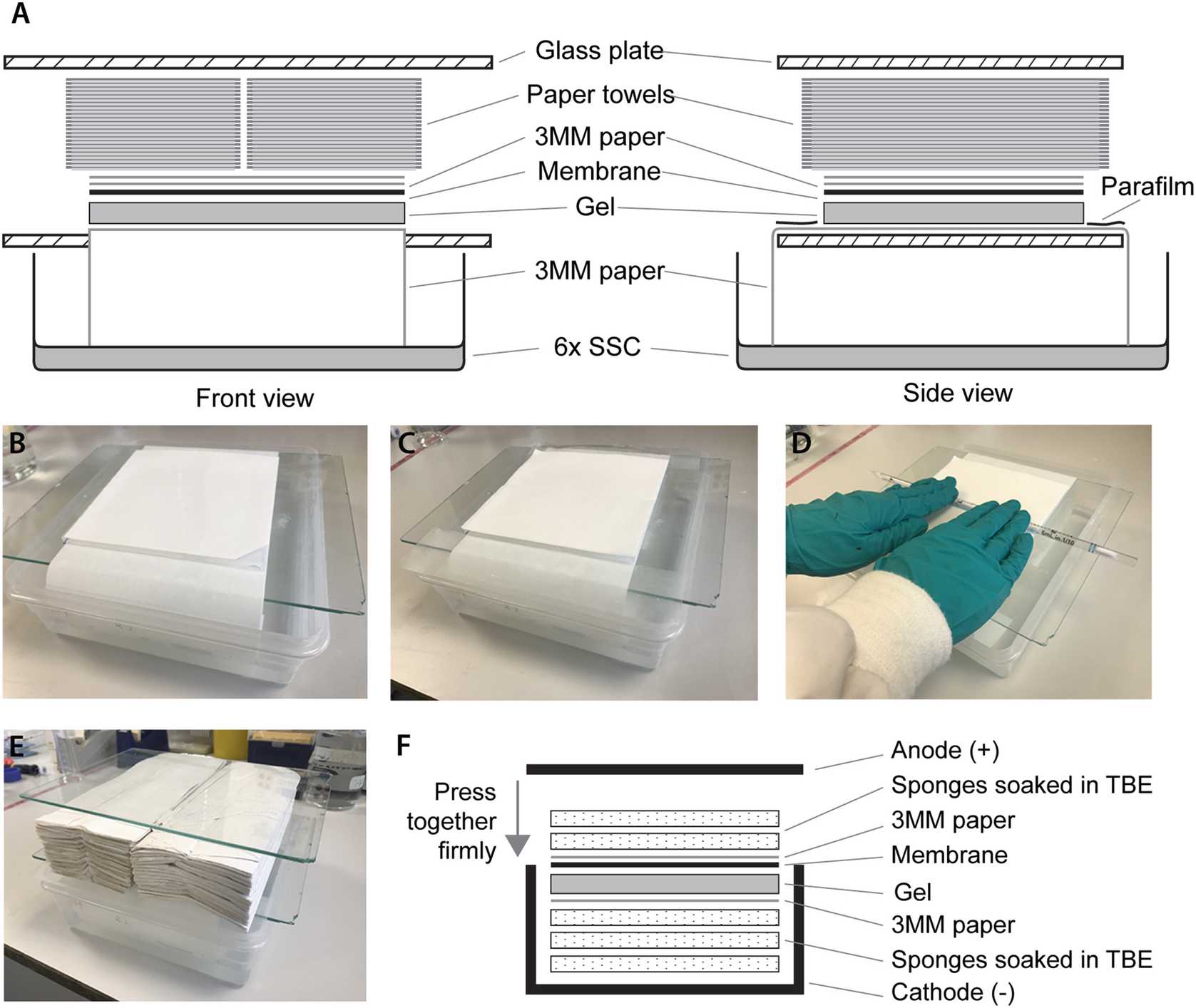
Put a glass plate over the top leaving a gap to fit the Whatman paper through into the 6× SSC.
Wet the long strip of Whatman paper with 6× SSC and place over the plate such that both ends are in the 6× SSC reservoir.
Very carefully flip the gel and place it onto the Whatman paper with the wells facing down.
Place the wetted membrane over the gel using the cut corner to track the orientation of the wells (see Fig. 1b).
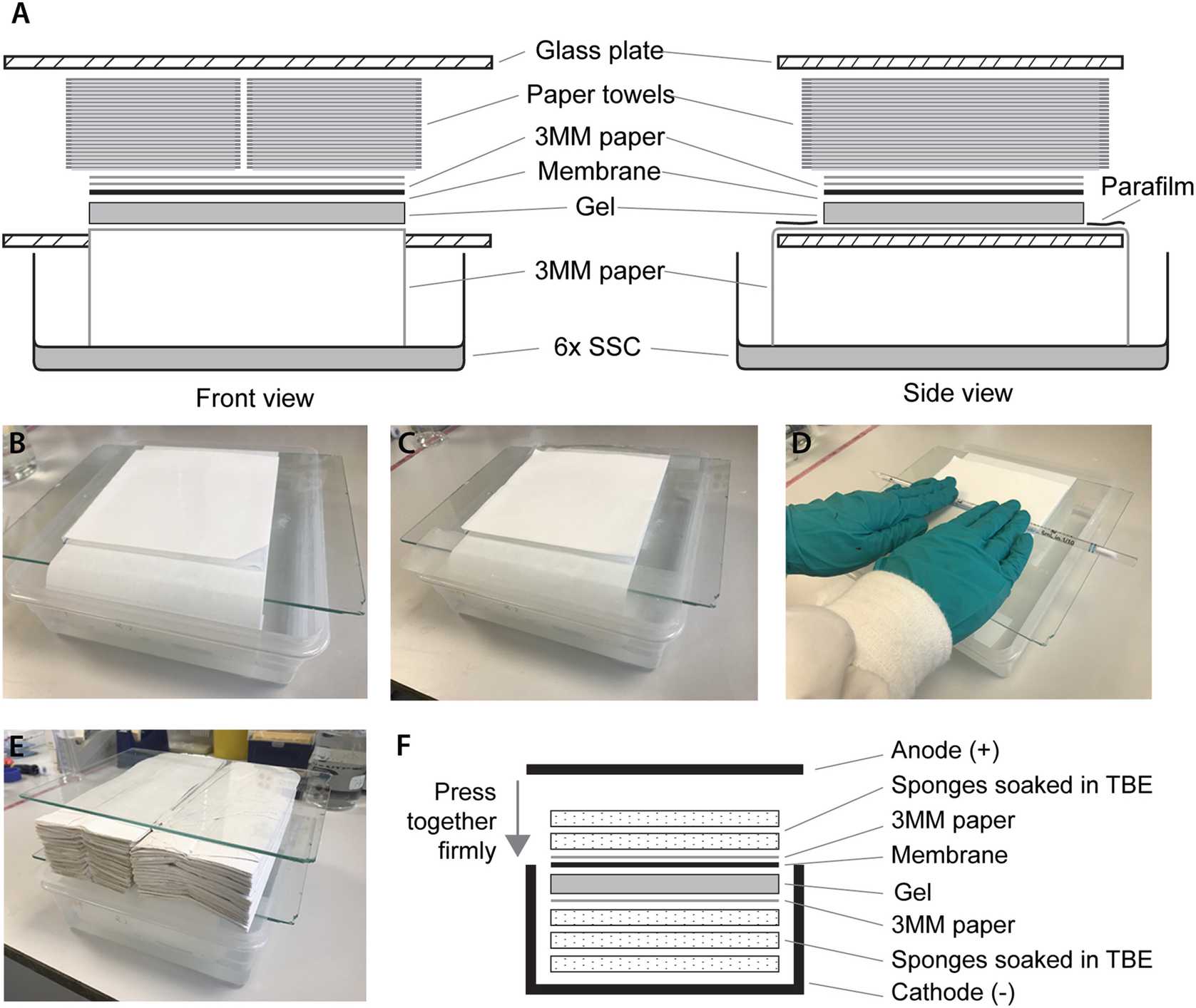
Place the two square pieces of Whatman paper on top of the membrane. Roll a Stripette over the gel “sandwich” to get rid of bubbles.
Place two strips of Parafilm covering the ends of the long strip of paper that are not covered by the gel (see Fig. 1c, d).
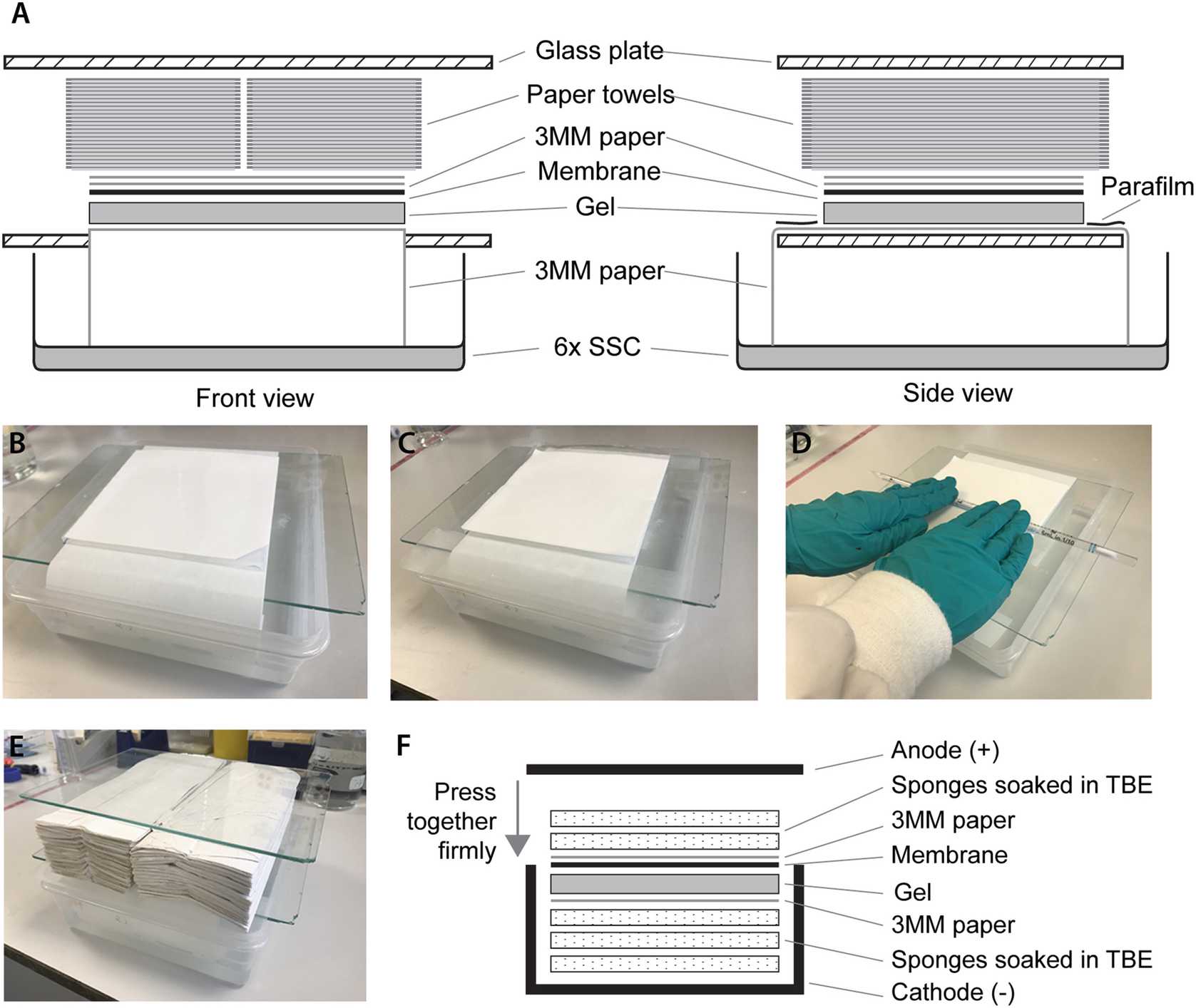
Place two stacks of paper towel of about 5 cm high next to each other over the “sandwich”.
Place another glass plate on top and leave the transfer apparatus overnight (see Fig. 1a, b, e).

Pick up the two pieces of Whatman paper and the membrane together and place on the floor of the cross-linker with the RNA side facing upward, enabling direct irradiation of the RNA by the ultraviolet bulbs. Irradiate using Auto-crosslink mode for a Stratalinker or 120,000 μJ/cm2 for other cross-linkers.
Wrap the membrane only in cling film and keep at 55Room temperature for probing on the same day or at -20°C for long-term storage.

