Synapse Staining - IHC - VGluT1 and PSD95 - Mouse Brain Sections
Justin T Savage, Juan Ramirez, Dolores Irala, Cagla Eroglu
Abstract
Instructions to stain for pre- and post-synaptic markers used to quantify the number of synapses in mouse brain. This protocol specifically labels VgluT1+ inputs which in the mouse cerebral cortex are intracortical. The post-synaptic marker is PSD95 which in the mouse cerebral cortex labels excitatory post-synapses. This protocol has been used to label synaptic compartments at several developmental ages and has been paired with standard confocal microscopy and super-resolution light microscopy (STED). The analysis is facilitated by an in-house ImageJ macro taking advantage of the diffraction limit of standard confocal microscopy to quantify synapses as the observed colocalization of pre- and post-synaptic markers.
Before start
Tissue must be prepared as follows:
-
Mice were anesthetized with a lethal dose of avertin (1.25% solution in water or 12.5mg/mL in water).
-
After mice are no longer responsive to toe pinch, they were transcardially perfused with 1X Tris Buffer Saline (TBS) until they run clear (~ 5 minutes).
-
Following transcardial perfusion with 1X TBS, mice were then perfused with 4% Paraformaldehyde (PFA) dissolved in 1X TBS for ~ 5 minutes (until stiff).
-
Finally, brains were dissected from the mouse and stored overnight (~12-18 hours) at 4 degrees Celsius in 4% PFA (enough to cover the brain).
-
The following day, brains were removed from PFA, rinsed with 1X TBS, and then stored at 4 degress Celsius in a 30% sucrose solution in 1X TBS.
-
The brains are stored until they sink to the bottom of their container and can then be frozen and cryosectioned.
Any alterations to tissue preparation should be tested empirically to be certain the resulting staining is still comparable to the staining resulting from the previously described tissue preparation.
STED Mounting media was made following the recipe here: https://nic.med.harvard.edu/resources/media/
In brief, the final solution is 50mM N-Propyl Gallate, 20 mM Tris, and 90% Glycerol in water.
Steps
Day 1: Buffer preparation
Prepare 50 mL of 0.2% Triton in 1X TBS ( TBST ) by combining 1 mL of Triton X-100 with 49 mL of 1X TBS. Vortex to mix well.
Prepare 7.5 mL of 5% Normal Goat Serum in TBST ( NGST ) by combining 375 μL of Goat serum with 7.125 mL of 0.2% TBST. Vortex to mix well. Any leftover NGST can be stored at 4 °C until it is needed for the duration of the staining protocol. Do not use NGST older than 1 week.
Prepare Primary Antibody Buffer ( 1° AB ) by aliquoting 2.5 mL of 5% NGST into a new tube. Add appropriate dilution of each antibody to your tube making sure to use new tips for each antibody. For 2.5 mL of 1° AB, add 0.34 μL of guinea pig anti-VGluT1 and 8.33 μL of rabbit anti-PSD95. Vortex to mix well. Centrifuge the 1° AB at max rpm at 4°C for 5 minutes. After completing the spin, store the 1° AB on ice or at 4 °C until ready to use.
Day 1: Primary antibody staining
Get a fresh 24 well plate, orient the plate as depicted in Figure 1 , and label the first three rows for TBST. Fill each well with 1 mL of TBST.
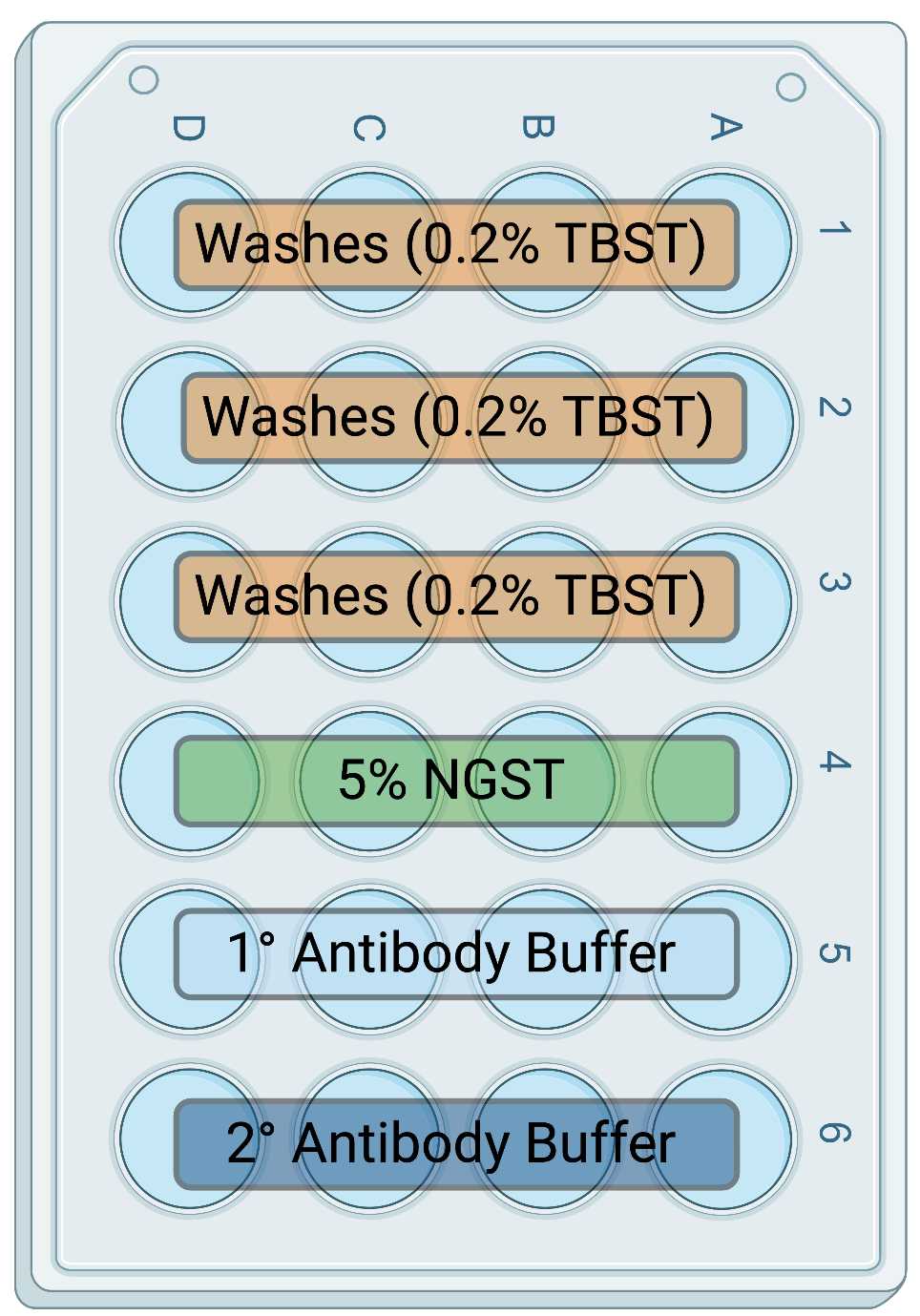
Using a flame-shaped glass pipette (as depicted in Figure 2 ), transfer up to 3 brain sections into one well to wash off OCT or glycerol storage buffer. Incubate at room temperature (RT) in this well for 5-10 minutes.

Transfer brain sections to the next wash well (keeping each sample within it's own column A,B,C, or D as depicted in Figure 1 ). Incubate at RT for 5-10 minutes.
Repeat step 6 and allow sections to incubate at RT for 5-10 minutes.
Aliquot 500 μL of 5% NGST into each well labeled for NGST.
Transfer brain sections to the NGST well and allow them to incubate at RT for 1 hour.
Aliquot 500 μL of 1° AB in each well labeled for 1° Antibody Buffer.
Transfer brains sections to the 1° AB and allow them to incubate at 4 °C overnight (12-18 hours)
Day 2: Buffer Preparation
Prepare Secondary Antibody Buffer ( 2° AB ) by aliquoting 2.5 mL of 5% NGST into a new tube. Add appropriate dilution of each secondary antibody to your tube making sure to use new tips for each secondary antibody. For 2.5 mL of 2° AB, add 12.5 μL of goat anti-guinea pig Alexafluor 647 and 12.5 μL goat anti-rabbit Alexafluor 488. Vortex to mix well. Centrifuge the 2° AB at max rpm at 4 °C for 5 minutes. After completing the spin, store the 2° AB on ice and covered or at 4 °C in the dark until ready to use.
Day 2: Secondary antibody staining
Vacuum off the old wash buffer from the first three rows and replace it with 1 ml of fresh 0.2% TBST per well.
Transfer the sections from the 1° AB solution to the first wash well. Allow them to incubate at RT for 5-10 minutes. Repeat this step twice by transferring the sections to the next wash well for a total of 3 washes.
Aliquot 500 μL of the 2° AB solution to the wells labeled for 2° AB. Transfer the sections to this well and incubate at RT for 2 hours (light protected).
After the 2 hour incubation, vacuum off the old wash buffer from the first three rows and replace it with 1 ml of fresh 0.2% TBST per well.
Transfer the sections from the 2° AB solution to the first wash well. Allow them to incubate at RT for 5-10 minutes. Repeat this step twice by transferring the sections to the next wash well for a total of 3 washes.
Mount Sections and Coverslip
Prepare each frosted glass slide by drawing a rectangle on it with a PAP pen and labeling it with the appropriate information.
Add 1 mL of 1X TBS to the slide you are currently mounting. Using the paint brush, carefully transfer the brain sections onto the slide and flatten into place.
Once the sections are in place, with a pipette carefully remove excess 1X TBS. Once the sections appear firmly set on the glass, remove residual 1X TBS using either a Kimwipe or a vacuum line. (Be careful not to touch the sections or damage them.)
When the sections are dried into place, but not fully dried (there should be not salt build-up and the sections should still be slightly opaque not transparent), add 70 uL of mounting media to your slide.
Carefully, place the coverslip onto the slide. Do not allow bubbles to buildup on the sections.
Once the coverslip is on the sections, seal the slide using the clear nail polish. You need to ensure the slides are totally sealed to prevent leakage of the mounting media, but do not cover your sections with nailpolish.
After the slides dry, either image immediately or store at 4 °C in the dark. Best practice is to image the slides within 48 hours after completing secondary to avoid fading of the secondary dye.

