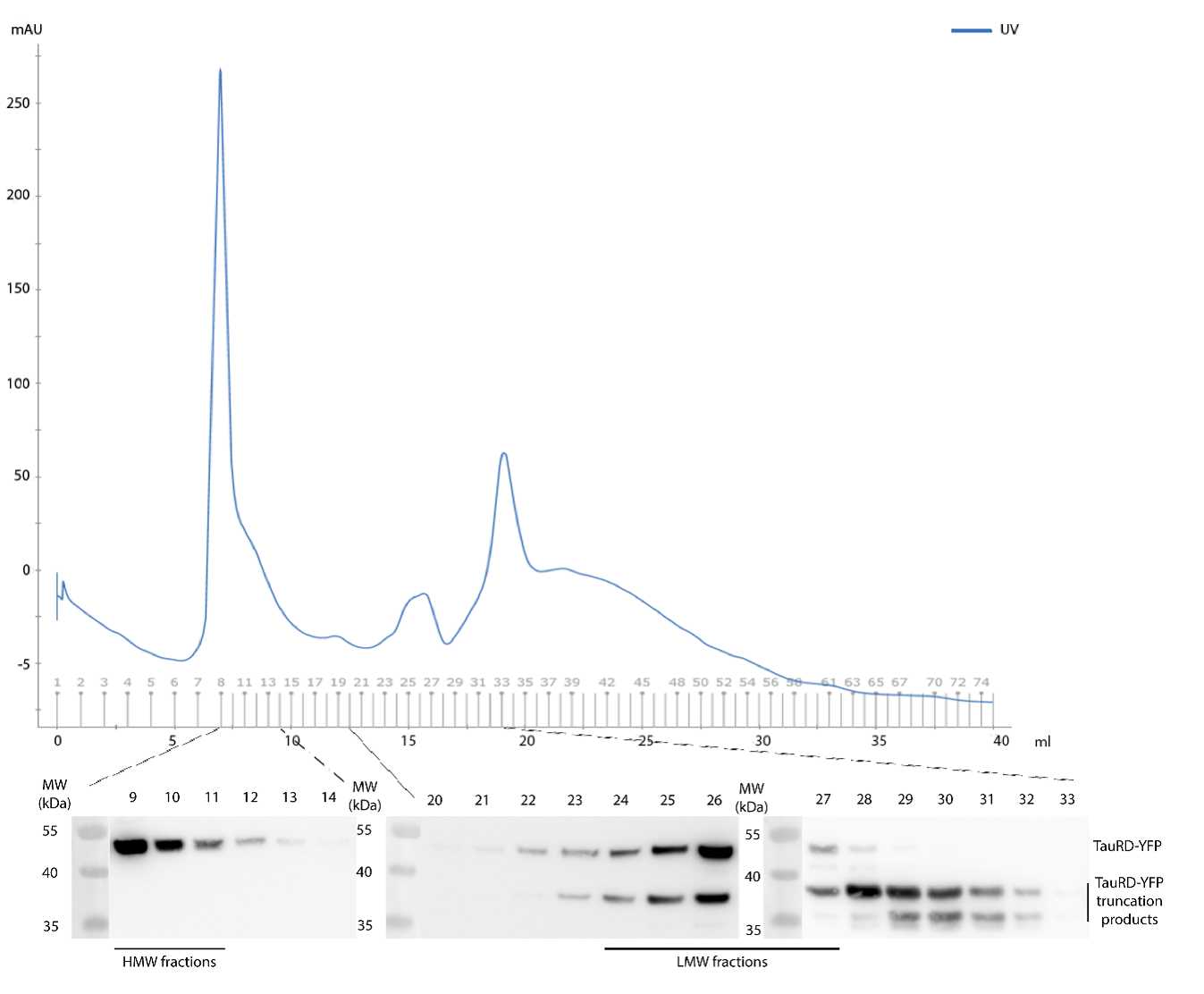
Size exclusion chromatography of cell lysates containing Tau aggregates
Patricia Yuste Checa, Itika Saha, F. Ulrich Hartl, Mark S. Hipp
Abstract
This protocolcan be used to fractionate Tau aggregates from cell lysates by size exclusion chromatography. The protocol was optimized using HEK293 cells stably expressing and propagating aggregates of Tau repeat domain, containing the disease-related mutations P301L and V337M, fused to YFP (Clone 10 from Sanders et al., Neuron, 2014). However, any cell line containing Tau aggregates can be used.
Steps
Lyse cell pellets with Triton buffer, Complete EDTA-free protease inhibitor cocktail (Roche) and benzonase for 20 min on ice. NOTE: Triton buffer: 0.05% Triton X-100/PBS or 1% Triton X-100/PBS (more efficient lysis) can be used to lyse cell pellets.
Clarify the lysates by centrifugation at 1,000 x g for 5 min at 4 °C and filter the supernatant with a PVDF 0.22 μm filter (Millex, #SLGVX13NL).
Quantify total protein by Bio-Rad Protein Assay (Bio-Rad) or Pierce BCA Protein Assay Kit (Thermo Fisher Scientific).
Load 3 mg total protein on a Superose 6 HR10/30 (GE 875 Healthcare) column previously equilibrated with PBS.
NOTES: Cells from a confluent T75 flask lysed with 800 µL lysis buffer results in ~ 8-10 mg/mL total protein. Superose 6 HR10/30 (GE Healthcare): Flow rate 0.5 mL/min; 1mL fractions up to void volume of the column and 0.5 mL fractions from void volume to the end.
Analyze and quantify individual fractions by Western blot. NOTES: Use 15 µl of each fraction to detect TauRD-YFP by immunoblotting with anti-GFP antibody (Roche #11814460001).
The corresponding fractions, HMW and LMW species, can be pooled and quantified (using purified TauRD-YFP as standard) by immunoblotting to perform other biochemical assays or cell-based Tau seeding assays.