Recombinant Human Proteoglycan Aggrecan-G1 Domain-induced Arthritis (GIA) Mouse Model
Katalin Olasz, Katalin Olasz, Ferenc Boldizsar, Ferenc Boldizsar
Abstract
The recombinant human proteoglycan aggrecan-G1 domain (rhG1)-induced arthritis (GIA) mouse model is a complex model of rheumatoid arthritis (RA). In GIA, autoimmune arthritis is induced by repeated intraperitoneal immunization of genetically susceptible BALB/c mice with the rhG1 antigen emulsified in the adjuvant dimethyldioctadecylammonium (DDA). This article describes the steps for producing and purifying the rhG1 antigen, the immunization protocol, methods for following the clinical picture of arthritis, and the evaluation of relevant laboratory parameters. In this model, the autoimmune arthritis develops stepwise, similar to RA: First is the preclinical stage (after the first immunization, days 0-20) with no sign of inflammation but detectable T and B cell activation; next, the stage of early arthritis (after the second immunization, days 21-41), where the first definitive signs of arthritis appear together with autoantibody production; and then the severe late-stage arthritis (after the third immunization, after day 42), which presents with massive inflammation of the limbs, leading to cartilage and bone destruction and finally ankylosis. The protocols described here provide sufficient information for investigators to use the GIA model to study different aspects of autoimmune arthritis. © 2024 The Authors. Current Protocols published by Wiley Periodicals LLC.
Basic Protocol : Induction of recombinant human proteoglycan aggrecan-G1 domain (rhG1)-induced arthritis (GIA)
Support Protocol 1 : Production of rhG1-Xa-mFc2a fusion protein with CHOK1 mammalian expression system
Support Protocol 2 : Purification of the rhG1-Xa-mFc2a fusion protein by affinity chromatography
Support Protocol 3 : Preparation of DDA adjuvant
Support Protocol 4 : Clinical assessment of arthritis
Support Protocol 5 : Measurement of serum antibody levels and cytokines
Support Protocol 6 : Measurement of rhG1-induced proliferation and cytokine production in spleen cell culture
Support Protocol 7 : Histological assessment of arthritic limbs
Support Protocol 8 : Evaluation of arthritis with micro-computed tomography
INTRODUCTION
Recombinant human proteoglycan (PG) aggrecan G1 domain (rhG1)-induced arthritis (GIA) is an experimental model for rheumatoid arthritis (Glant et al., 2011). GIA develops upon immunization of genetically susceptible BALB/c mice with the mixture of rhG1 and DDA adjuvant, which leads to the activation of cross-reactive T- and B cells that not only recognize the G1 domain of the recombinant protein, but also react with the self-cartilage PG of the immunized mice. This autoimmune arthritis model shares several features with RA: (i) The clinical picture develops stepwise and leads to progressive and irreversible destruction of the cartilage and bone tissue of the small joints; (ii) autoantibodies against mouse PG aggrecan, rheumatoid factor (RF), and anti-CCP appear in the serum; (iii) inflammatory cytokines are produced (IL-6, TNFα); and (iv) differentiation and activation of Th1 and Th17 cells occur (Glant et al., 2011). In the predecessor of GIA model, purified human cartilage PG aggrecan was used to induce autoimmune arthritis (PGIA; Glant et al., 1987). Although the GIA and PGIA models share several features, GIA is superior to PGIA: the recombinant antigen can be produced in a simple in vitro cellular expression system giving an unlimited source and superb quality, whereas in PGIA the antigen collection and purification was time consuming and limited.
Here we describe the induction of GIA in BALB/c mice (see Basic Protocol) and furthermore provide protocols for the production and purification of the rhG1 antigen (see Support Protocols 1 and 2, respectively), preparation of the DDA adjuvant (see Support Protocol 3), clinical assessment of the arthritis (see Support Protocol 4), measurement of serum antibodies and cytokines (see Support Protocol 5), measurement of proliferation and cytokine production in antigen stimulated spleen cell cultures (see Support Protocol 6), and finally, histological and CT analysis of the inflamed limbs (see Support Protocols 7 and 8, respectively).
NOTE : All protocols involving animals must be reviewed and approved by the appropriate Animal Care and Use Committee and must follow regulations for the care and use of laboratory animals.
STRATEGIC PLANNING
The induction of GIA requires three intraperitoneal injections of rhG1 antigen and DDA adjuvant given every three weeks (on days 0, 21, and 42), and severe arthritis will develop in the mice starting 45-48 days after the first injection.
NOTE : All protocols involving animals must be reviewed and approved by the appropriate Animal Care and Use Committee and must follow regulations for the care and use of laboratory animals.
Basic Protocol: INDUCTION OF RECOMBINANT HUMAN PROTEOGLYCAN AGGRECAN-G1 DOMAIN (rhG1)-INDUCED ARTHRITIS (GIA)
Repeated intraperitoneal immunization of genetically susceptible BALB/c mice with the rhG1 antigen using DDA adjuvant leads to progressive autoimmune arthritis sharing several similarities with RA. In GIA the differentiation and activation of autoreactive T- and B cells is critical, leading to the production of autoantibodies, inflammatory cytokines, and rheumatoid factor (RF). The severe, progressive autoimmune inflammation of the joints leads to bone and cartilage erosion and ankyloses. Importantly, as in RA, anti-CCP antibodies are detectable in mice with GIA.
Materials
-
Lyophilized rhG1-Xa-mFc2a antigen (see Support Protocols 1 and 2)
-
Dimethyldioctadecylammonium (DDA) powder
-
Phosphate-buffered saline (PBS), sterile
-
5- to 6-month-old female BALB/c mice
-
50-ml sterile tube with cap
-
Vortex
-
2-ml sterile syringe
-
23-G sterile syringe needle
Preparation of antigen
1.In planning the experiment, plan to use the following amounts of reagents per mouse for arthritis induction:
- 40 μg rhG1-Xa-mFc2a;
- 2 mg DDA;
- 300 μl sterile PBS.
2.Measure the required amount of lyophilized rhG1-Xa-mFc2a into a sterile 50-ml tube with cap and dissolve it in one-third of the sterile PBS.
3.Prepare the required amount of DDA in a sterile 50-ml tube with cap, dissolve it in one-third of the sterile PBS, and prepare DDA emulsion as described in Support Protocol 3.
4.When the emulsion has completely cooled, mix it thoroughly with the dissolved rhG1-Xa-mFc2a protein and then add the remaining one-third of the sterile PBS.
Immunization of mice and assessment of arthritis
5.Aspirate the antigen-adjuvant mixture into a sterile 2-ml syringe and inject 300 μl of it intraperitoneally into each mouse.
6.To induce arthritis, perform two more immunizations three weeks apart, so that 21 days pass between each immunization.
7.Begin clinical scoring (assessment of arthritis, as described in Support Protocol 4) as needed; this generally starts after the second immunization (i.e., after day 21). Mice are scored three times per week. Additionally, limbs can be measured using a digital caliper as described in Support Protocol 4.
8.Additionally, at any point in the experiment, micro-CT imaging can be performed from the limbs to assess the joints and bone as described in Support Protocol 8.
Assessment of the immune response
9.Two weeks after the third immunization (when peak arthritis severity and immune response are usually reached), terminate the experiment by euthanizing the mice. Collect blood in microcentrifuge tubes and let them coagulate, and then centrifuge the samples for three rounds at ∼16,000 rcf, room temperature. The serum obtained in this manner can be used for the following measurements:
-
To determine the IgG1 and IgG2a antibodies produced against the rhG1 antigen used for immunization (anti-rhG1 antibodies) as described in Support Protocol5, steps 1a-13a;
-
To determine the IgG1 and IgG2a autoantibodies produced against the mouse's own proteoglycan as described in Support Protocol5, steps 1b-11b;
-
To determine serum cytokine (IL-1β, IL-4, IL-6, IL-17, IL-23, IFNγ, and TNFα) levels as described in Support Protocol5, steps 1c-7c and 1d-4d.
10.If desired, isolate spleens under sterile conditions for determination of cell proliferation and cytokine production of spleen cells upon antigen stimulus (Support Protocol 6). Antibody and cytokine levels, as well as cell proliferation, correlate with arthritis severity, and thus these measurements provide valuable additional information alongside severity scores. The cytokines IL-1β, IL-4, IL-6, IL-17, IL-23, IFNγ, and TNFα are routinely measured in GIA because they play roles in the pathogenic process; however, these can be complemented by other cytokines of interest in special cases.
11.If desired, isolate inflamed limbs for histological assessment (Support Protocol 7).
Support Protocol 1: PRODUCTION OF rhG1-Xa-mFc2a FUSION PROTEIN WITH CHOK1 MAMMALIAN EXPRESSION SYSTEM
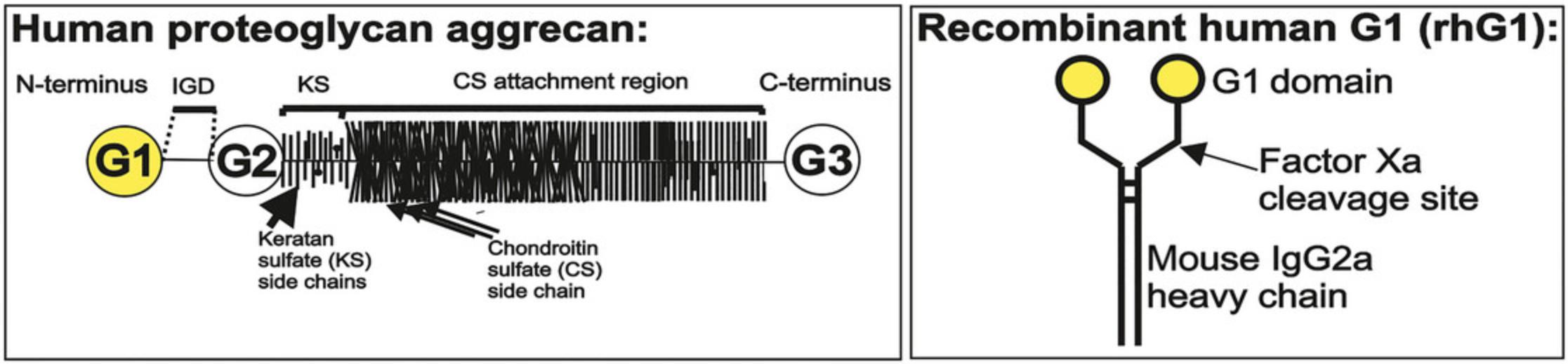
The basis of the GIA model is the induction of arthritis with rhG1-Xa-mFc2a fusion protein (Murad et al., 2005). This fusion protein consists of the G1 domain of the human PG aggrecan fused with a mouse IgG2a Fc portion (Fig. 1). Repeated intraperitoneal immunizations with rhG1-Xa-mFc2a protein initiate the production of cross-reactive autoantibodies in genetically predisposed BALB/c mice. We use a mammalian expression system in order to obtain a sufficient amount of rhG1-Xa-mFc2a protein for arthritis induction. CHOK1 cells transfected with the rhG1-Xa-mFc2a Lonza construct can be used for the reliable production of the recombinant protein required for arthritis induction (Glant et al., 2011).
Materials
-
CHOK1 cell line transfected with the rhG1-Xa-mFc2a Lonza construct (available upon personal request from the authors)
-
DMEM with 4.5 g/L glucose and L-glutamine, supplemented with 10% (v/v) FBS and 1% (v/v) antibiotics stock solution
-
Antibiotic stock solution: 107 U/L penicillin and 10 g/L streptomycin
-
Phosphate-buffered saline (PBS), sterile
-
Trypsin solution (100 ml PBS with 0.25 g trypsin and 20 µl of 0.5 M EDTA), sterile
-
5 L of serum-free CHO cell culture medium: ProCHOTM 5 Protein-free CHO Medium, 1 L (Lonza, cat. no. BELN12-766Q) with HEPES and 0.1% Pluronic® F-68, without L-glutamine, phenol red, hypoxanthine or thymidine
-
Sodium azide
-
Sterile hood
-
15- and 50-ml conical centrifuge tubes with cap, sterile
-
Centrifuge: Eppendorf 5804R or similar
-
37°C, 5% CO2 incubator
-
Sterile petri dishes: Falcon® 150 mm TC-Treated Cell Culture Dish with 20 mm Grid, 10/Pack, 100/Case, Sterile and 100 mm BioLite™ Cell Culture Treated Dishes
-
Pipettor Turbo-Fix
-
25-ml serological pipets, sterile
-
Filtration unit: Stericup® with Millipore Express® PLUS (PES), membrane pore size 0.22 μm, 500 ml
-
Cryovials: Thermo Scientific™ Nalgene™ System 100™ Cryogenic Vials (optional)
NOTE : During rhG1-Xa-mFc2a production, cell manipulation should be performed in a sterile hood, using sterile equipment and chemicals.
1.Thawing the cells (supplied as a freezing capsule containing ∼4 × 106 CHOK1 cells transfected with rhG1-Xa-mFc2a in 1 ml of freezing medium, stored in liquid nitrogen) in a 37°C water bath. Transfer the cells, together with the freezing medium, into a sterile 15-ml conical centrifuge tube, and then add 10 ml DMEM with 10% FBS and 1% antibiotic. Mix by inverting the tube two or three times.
2.Centrifuge the cells 5 min at 1000 rpm, room temperature, in the Eppendorf 5804R centrifuge (183 rcf) and then carefully remove the supernatant. Gently vortex the pellet and repeat the wash by adding another 10 ml DMEM/10% FBS with 1% antibiotic and centrifuging again for 5 min at 1000 rpm (183 rcf), room temperature.
3.Resuspend the cells in 15 ml of DMEM/10% FBS with 1% antibiotic. Transfer the resuspended cells to a sterile 100-mm-diameter petri dish and place it in the 37°C, 5% CO2 incubator.
4.When the cells reach confluence, aspirate the supernatant with a pipettor and then wash the cells with 15 ml sterile PBS.
5.Detach adhered cells by adding 3 ml sterile trypsin solution and incubating in 37°C incubator for 5 min.
6.Collect the detached cells in sterile 15-ml conical centrifuge tubes. Add 10 ml DMEM/10% FBS and 1% antibiotic and then centrifuge the cells for 5 min at 1000 rpm (183 rcf). Gently aspirate the supernatant and then resuspend the cells in 15 ml DMEM/10% FBS with 1% antibiotic.
7.Divide the 15 ml of cell suspension five sterile 100-mm-diameter petri dishes (3 ml of cell suspension per dish). Add 12 ml of DMEM/10% FBS with 1% antibiotic to each 3 ml of cell suspension in the petri dishes and incubate in 37°C incubator until cells reach confluence.
8.Repeat steps 5-8 (this will result in 25 petri dishes of CHOK1 cells).
9.Repeat steps 5-6.Collect the cells from each petri dish into two sterile 50-ml centrifuge tubes and then centrifuge tubes 5 min at 1000 rpm (183 rcf), room temperature. Discard and aspirate the supernatant, and then resuspend the cells in 50 ml serum-free CHO cell culture medium per tube. With a pipettor, transfer 2 ml each of cell suspension to 50 sterile 150-mm-diameter petri dishes, and reconstitute the medium volume in each petri dish by adding 28 ml of serum-free CHO medium. Place the petri dishes in the 37°C incubator.
10.Harvest the supernatant in two rounds on day 3 and day 5.After each harvest, pool the supernatants, filter with a Millipore Express® PLUS (PES) Stericup with a 0.22-μm-pore-size membrane, and store at 4°C; then add 30 ml fresh serum-free CHO medium to the cells in each petri dish.
11.After two rounds of harvesting, discard the cells along with the petri dishes; you should have ∼5 L of pooled and filtered supernatant. Supplement the pooled supernatant with 0.1% (w/v) sodium azide to avoid contamination.
12.The supernatant can be stored for up to 2 weeks at 4°C before use. However, to prevent protein degradation, we recommend proceeding with Support Protocol 2 as soon as possible.
Support Protocol 2: PURIFICATION OF THE rhG1-Xa-mFc2a FUSION PROTEIN BY AFFINITY CHROMATOGRAPHY
The collected supernatant ideally contains several milligrams of rhG1-Xa-mFc2a fusion protein per liter, which can be purified on a protein G column using affinity chromatography (Glant et al., 2011). Because this is produced in serum-free CHO medium, the purification is not complicated by immunoglobulin of other origin. Either a pre-filled column can be purchased or a gravity-flow column and protein G Sepharose beads can be purchased separately and used. In the latter case, the beads are loaded into the column before use; we describe the procedure below.
Materials
-
5 liters pooled, filtered supernatant from CHOK1 cells expressing rhG1-Xa-mFc2a (Support Protocol 1)
-
Protein G Sepharose™ 4 Fast Flow Affinity Chromatography beads
-
Phosphate-buffered saline (PBS) with 0.1% (w/v) azide (the binding buffer provided by the manufacturer can also be used)
-
Elution buffer: 0.1 M glycine, pH 2.7
-
Neutralizing buffer: 1 M Tris·Cl, pH 9.0
-
Affinity chromatography gravity-flow columns (made of high-purity polypropylene with a very low adsorption to various biomolecules) together with additional equipment
-
5-liter Erlermeyer flasks
-
10-ml glass test tubes
1.Assemble the gravity-flow column for affinity chromatography, loading the protein G Sepharose beads into the column so that the beads settle in the column without bubbles above the filter.
2.Wash the column three times with 1 column volume PBS with 0.1% (w/v) azide.
3.Apply the supernatant to the column. The flow rate required for protein binding is at least 7 s/drop. If all 5 liters of the supernatant can be pooled in a sufficiently large flask, the assembled system can be placed in a cold room overnight. In this case, the flow rate must be set so that it is no faster than 7 s/drop and so that the entire amount of supernatant will not flow through the column before the next morning, to prevent the protein G Sepharose beads from drying out.
4.When all the supernatant has flowed through the column, wash the column again three times with 1 column volume PBS with 0.1% azide.
5.Prepare an appropriate number of clean 10-ml glass test tubes: one per milliliter of protein G Sepharose beads used, plus one test tube in which the PBS with 0.1% azide on the column is collected. For example, if working with 10 ml protein G Sepharose beads, prepare at least 11 test tubes.
6.After collection of the washing buffer has finished, add elution buffer in a volume equal to the volume of the protein G beads and collect 1-ml fractions in the glass test tubes prepared in step 5.
7.Add 1 M Tris·Cl, pH 9.0 (200 µl/ml eluted fraction), to each fraction.
8.Regenerate the column by washing it three times with 1 column volume PBS with 0.1% azide.
9.Measure the protein content of the fractions using a spectrophotometer, and then pool the fractions containing the protein in large quantities.
10.Buffer exchange the eluted fractions after dialysis against double-distilled water.
11.Concentrate the protein solution after dialysis by lyophilization.
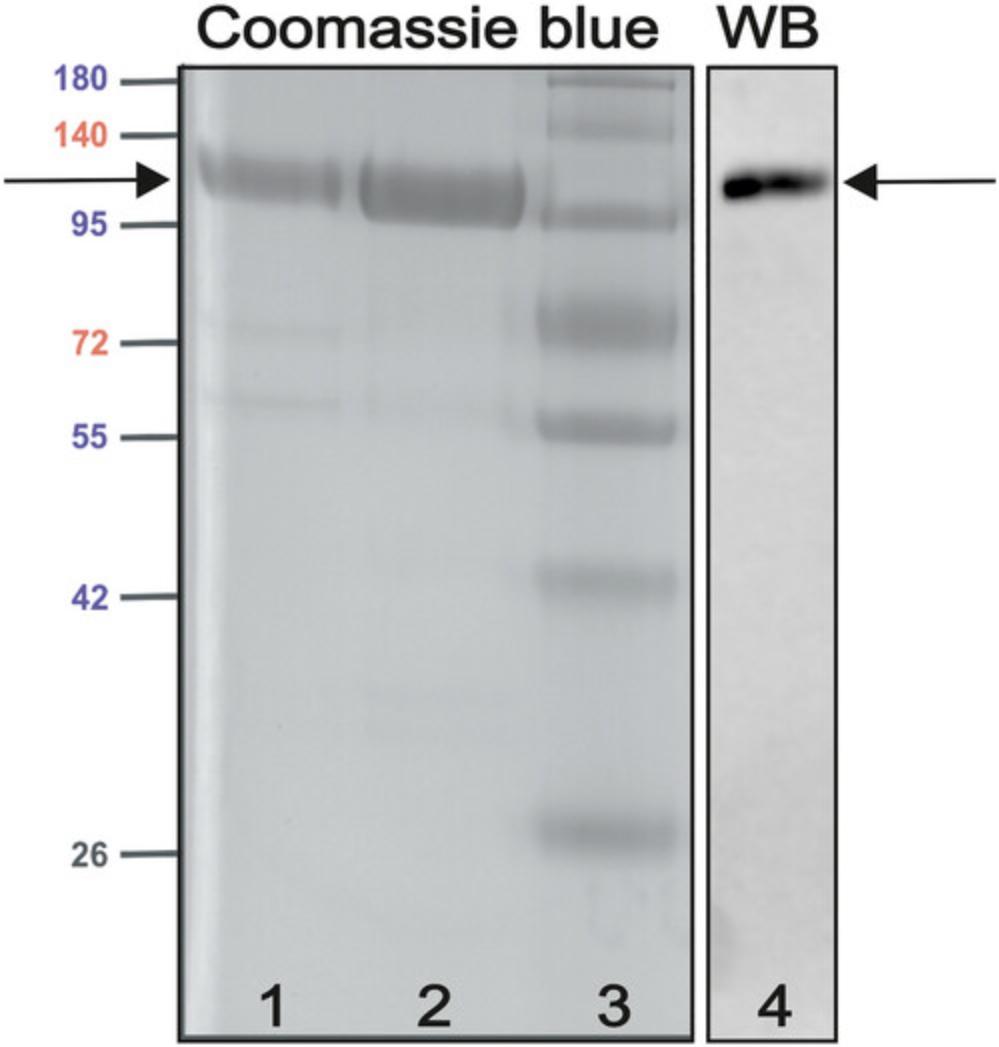
12.Quality assessment of the rhG1 antigen can be done using SDS-PAGE on a 10% gel followed by Coomassie blue staining (Fig. 2). The complete rhG1 molecule migrates between the 95- and 140-kDa molecular weight markers. For specific detection of the rhG1, a western blot with the G18 monoclonal antibody, specific for the G1 domain, can be used (Fig. 2).
Support Protocol 3: PREPARATION OF DDA ADJUVANT
Compared to other adjuvants, DDA is a moderately strong adjuvant for humoral immune responses and a strong adjuvant for cell-mediated (mainly DTH-type hypersensitivity) immune responses (Gordon et al., 1980; Katz et al., 1991; Hilgers & Snippe, 1992). It is also a common ingredient used in experimental immunization of laboratory animals and in vaccines used in veterinary medicine (Katz et al., 1991; Katz et al., 1996). As compared to the previously used complete Freund's adjuvant, DDA administered intraperitoneally during immunizations causes much fewer adhesions in the peritoneum, thus facilitating the removal of the spleen at the end of the experiment (Anita Hanyecz et al., 2004).
Materials
-
Dimethyldioctadecylammonium (DDA) powder
-
Phosphate-buffered saline (PBS), sterile
-
50-ml sterile tube with cap
-
Vortex
-
Microwave oven
-
Styrofoam box and ice
1.Measure 2 mg/mouse of DDA powder into a sterile 50-ml tube, add 100 μl/mouse sterile PBS, and thoroughly vortex.
2.Place the tube with the DDA-PBS mixture in the microwave oven with the cap closed semi-loosely and heat to boiling. Care must be taken because the mixture foams as a result of boiling. It is not advisable to prepare more than 25 ml of DDA adjuvant in a 50-ml tube, so when working with a larger amount, prepare it in several aliquots.
3.Before the mixture boils out of the tube, turn off the microwave, tighten the cap, and vortex it thoroughly; then place the tube in a Styrofoam box filled with ice.
4.After the mixture has cooled completely, repeat steps 3 and 4 two more times. Following boiling and thorough mixing, a thick, white, opalescent emulsion should be obtained.
Support Protocol 4: CLINICAL ASSESSMENT OF ARTHRITIS
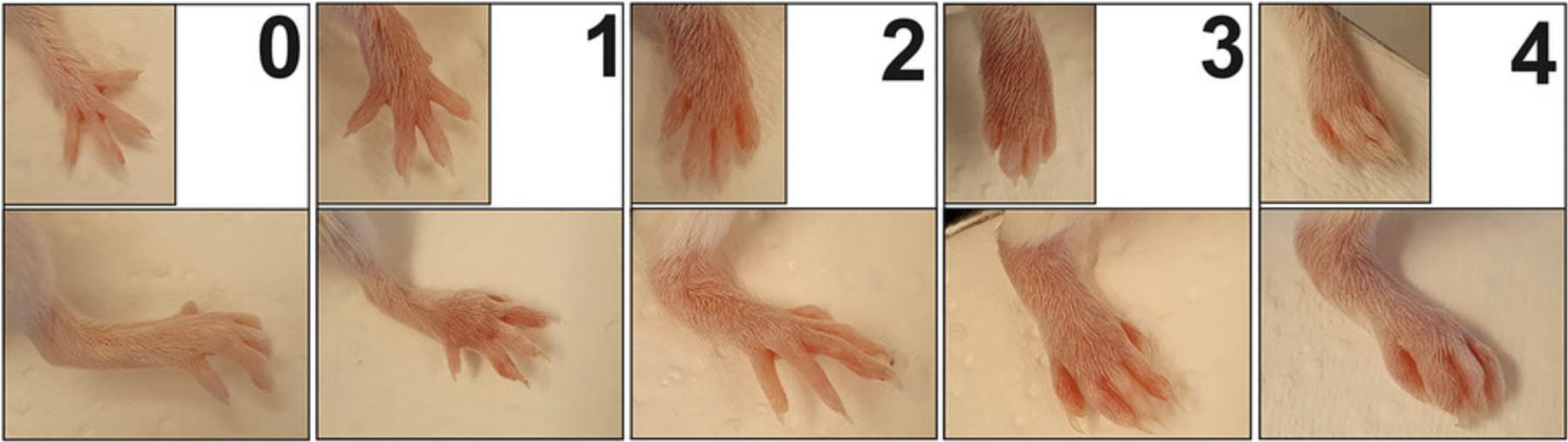
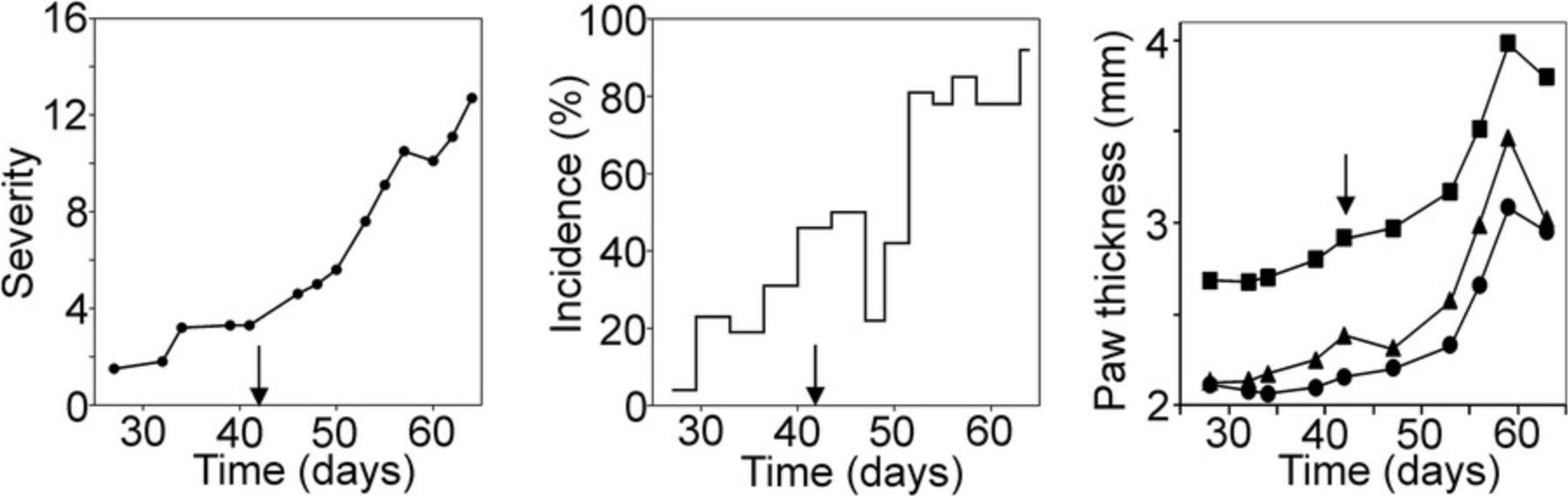
GIA is a readily detectable and visible disease model, so autoimmune arthritis can be easily examined visually (see steps 1a-3a below). A scoring system is used to describe the severity of inflammation (Table 1 and Fig. 3). Scoring includes examination of the front and hind paws of the mouse, with each leg receiving a score between 0 and 4 according to the degree of inflammation (Table 1 and Fig. 3). The sum of the scores gives the cumulative score (maximum 16; Fig. 4). In addition to this visual scoring, the thickness of the limbs can also be easily measured with a digital caliper (see steps 1b-4b below; Fig. 4). The inflammation usually starts in the digits and eventually affects the ankles and wrists. The inflammation can appear on all four limbs, but sometimes only the hind legs may be affected.
| Score | Pathology |
|---|---|
| 0 | No signs of erythema and swelling |
| 1 | Erythema and mild swelling limited to the mid-paws and fingers, but not affecting the ankles/wrists |
| 2 | Erythema and mild to moderate swelling affecting the ankle/wrist |
| 3 | Severe swelling and erythema of the entire limb (fingers, mid-paws, and ankle/wrist) without ankylosis |
| 4 | Ankylosis of the joints with ulnar deviation |
Materials
- BALB/c mice with GIA (Basic Protocol 1)
- Digital caliper
Visual assessment of arthritis
1a. Hold the mouse securely to ensure easy access to the paws. Because clinical assessment of individual paws includes the physical examination of the limbs (moving and bending of joints), which can cause discomfort and pain for the mouse, perform the examination quickly and gently.
2a. Evaluate all four limbs, beginning with the front limbs, following the scoring system outlined in Table 1.
3a. As arthritis typically manifests after the second immunization, start the scoring 2 days after the second immunization and repeat it three times a week until the termination of the experiment.
Measurement of limb thickness with a digital caliper
1b. Hold the mouse in a position that facilitates paw measurements with the caliper.
2b. Average the measurements taken for the two front paws to determine front leg thickness.
3b. For the hind legs, measure the paw thickness at the middle paw and separately measure the ankle thickness. Average the measurements for both hind legs to obtain paw and ankle thickness data.
4b. Start the measurements 7 days after the second immunization and repeat it twice a week until the end of the experiment. Note: It is advisable to take some baseline measurements for each mouse before the immunization starts to serve as reference values.
Support Protocol 5: MEASUREMENT OF SERUM ANTIBODY LEVELS AND CYTOKINES
When the arthritic mice are euthanized, blood is collected in microcentrifuge tubes, allowed to coagulate, and subjected to three rounds of centrifugation to obtain serum. This serum can be used for the measurement of the levels of antibodies against rhG1 of and mouse proteoglycan and cytokine (IL-1β, IL-4, IL-6, IL-17, IL-23, IFNγ, TNFα) concentrations (Table 2).
| Serum antibodya | Mean | SEM |
| Anti-rhG1 IgG1 | 1.4 | 0.4 |
| Anti-mPG IgG1 | 1.6 | 0.1 |
| Anti-mPG IgG2a | 0.5 | 0.1 |
| Serum cytokineb | ||
| IL-1β | 475.4 | 101.8 |
| IL-4 | 857.3 | 136.6 |
| IL-6 | 351.2 | 72.9 |
| IL-17 | 1085.0 | 387.3 |
| Il-23 | 759.4 | 443.7 |
| IFNγ | 2004.4 | 592.7 |
| TNFα | 4005.1 | 100.1 |
- a
Presented as OD values measured at 492 nm.
- b
Presented as concentration values (pg/ml).
-
Mean and SEM values were calculated from the data of 9 mice.
Measurement of rhG1-Specific Antibody Levels by Indirect ELISA
Materials
- Sera of BALB/c mice with GIA (Basic Protocol, step 9)
- Cleavage/capture buffer: 100 mM NaCl/50 mM Tris·Cl/5 mM CaCl2, pH 8.0
- Factor Xa Cleavage Capture Kit (Novagen, cat. no. 69037-3)
- Standard coating buffer: aqueous solution of 1.58 g anhydrous Na2CO3 + 2.94 g NaHCO3/1000 ml, pH 9.5
- Nonfat dry milk powder
- Phosphate-buffered saline (PBS)
- Washing buffer: 0.5% (v/v) Tween in PBS (PBST)
- Standard serum sample (optional): hyper-immunized arthritic mouse serum containing 0.2 mg/ml a-hG1 IgG1 and 0.2 mg/ml IgG2a, obtained by pooling serum from multiple mice with GIA
- Anti-mouse-IgG1 antibody conjugated with HRPO (BD Pharmingen™ HRP Rat Anti-Mouse IgG1, cat. no. 559626)
- Anti-mouse-IgG2a antibody conjugated with HRPO (BD Pharmingen™ HRP Rat Anti-Mouse IgG2a, cat. no. 553391)
- Orthophenylenediamine (OPD) chromogen (Thermo Fisher Scientific, cat. no. 34005)
- 30% (v/v) H2O2
- 3,3′,5,5′-Tetramethylbenzidine (TMB; BD OptEIA™ TMB Substrate Reagent Set, BD OptEIA™ TMB Substrate Reagent Set, cat. no. 555214)
- 4 M H2SO4 or HCl
- Nunc Maxisorp 96-well ELISA plates
- Automatic ELISA microplate reader
1a. Reconstitute (rehydrate) the dialyzed, lyophilized rhG1-Xa-mFc2a in cleavage/capture buffer, and then incubate it with factor Xa (from the Novagen Factor Xa Cleavage Capture Kit) overnight (16 hr) at room temperature (∼20°C) to allow cleavage to proceed. Allow the enzyme to absorb to Xarrest Sepharose (included in the kit) for 5 min at room temperature and then spin it in microcentrifuge tubes for 3 min at 2000 rcf, room temperature. The IgG2a-Fc fragment (from supernatant) can be removed by protein G Sepharose chromatography. The purified rhG1 protein (fraction not bound to protein G) can be used for coating.
2a. Coat Nunc Maxisorp 96-well plates with 0.1 μg rhG1/100 μl of coating buffer/well for measuring anti-rhG1-IgG1 isotype antibodies or with 0.2 μg rhG1/100 μl/well for anti-rhG1-IgG2a isotype assay. Incubate the plates overnight at room temperature (or 1-2 days at 4°C) in a humidified atmosphere (seal plates or wrap with wet paper towels and aluminum foil).
3a. Add 200 μl/well of 1.5% (w/v) nonfat dry milk dissolved in PBS (pH 7.2-7.4) and let stand at room temperature for 1 hr to block the plates (free binding sites).
4a. Wash plates 4-6 times with washing buffer (PBST).
5a. Perform a serial dilution of the standard sample in PBS from 1:3200 to 1:16,000.
6a. For the serum samples of unknown antibody levels, dilute the samples in PBS as follows:
- Anti-rhG1-IgG1 : 4000-16,000.
- Anti-rhG1-IgG2a : 3200-8000.
7a. Pipet 100 μl/well of these dilutions from the standard and unknown samples, in duplicate, into wells of 96-well plates. Incubate plates 2 hr at room temperature.
8a. Wash the plates with washing buffer 4-6 times.
9a. Add 100 μl secondary antibody diluted in PBS as follows:
- Anti-mouse IgG1-HRP: 1:4000.
- Anti-mouse IgG2a-HRP: 1:2000.
Incubate the plates 2 hr at room temperature.
10a. Wash the plates extensively by washing the plates six times, allowing them to stand with PBST for a few minutes between each washing cycle.
11a. Develop the reactions:
- For anti-rhG1-IgG1 : Mix 20 mg OPD in 60 ml PBS, add 10 μl concentrated (30%) H2O2 and pipet 100 μl of this mixture into each well.
- For anti-rhG1-IgG2a : Freshly mix BD OptEIA™ A and B TMB substrate components 1:1 (v/v) and pipet 100 μl of this mixture into each well.
12a. Stop the color reaction by adding 25 μl of 4 M H2SO4 or HCl, and read the plates with an automatic ELISA reader at the following wavelengths:
- Anti-rhG1-IgG1 (with OPD): 490 nm.
- Anti-rhG1-IgG2a (with TMB): 450 nm.
13a. Prepare a standard curve based on the standard sample. Use/select the sample dilution that is in the linear range of the standard (control) sample.
Measurement of Serum Autoantibody Levels (Anti-Mouse PG) by Indirect ELISA
Materials
- Sera of BALB/c mice with GIA (Basic Protocol, step 9)
- Nunc Maxisorp 96-well ELISA plates
- Standard coating buffer: aqueous solution of 1.58 g anhydrous Na2CO3 + 2.94 g NaHCO3/1000 ml, pH 9.5
- Mouse proteoglycan (PG), lyophilized and dissolved in sterile phosphate-buffered saline (PBS) to obtain a 1 mg/ml stock solution
- Washing buffer: 0.5% (v/v) Tween in PBS (PBST)
- Blocking buffer: 1% (w/v) nonfat dry milk powder in PBS
- PBS, sterile
- Anti-mouse-IgG1 antibody conjugated with HRPO (BD Pharmingen™ HRP Rat Anti-Mouse IgG1, cat. no. 559626)
- Anti-mouse-IgG2a antibody conjugated with HRPO (BD Pharmingen™ HRP Rat Anti-Mouse IgG2a, cat. no. 553391)
- Orthophenylenediamine (OPD) chromogen
- 30% (v/v) H2O2
- 3,3′,5,5′-Tetramethylbenzidine (TMB; BD OptEIA™ TMB Substrate Reagent Set, BD OptEIA™ TMB Substrate Reagent Set, cat. no. 555214)
- 4 M H2SO4 or HCl
- Automatic ELISA microplate reader
1b. Coat ELISA plates with 0.1 μg mouse PG/100 μl carbonate coating buffer per well overnight at 4°C in a humidified atmosphere (seal plates or wrap with wet paper towel and aluminum foil).
2b. Wash the plates four times with 300 μl/well washing buffer (PBST).
3b. Block nonspecific binding sites with 300 μl/well blocking buffer (1% nonfat dry milk in PBS) for 1 hr at room temperature.
4b. Repeat washing (step 2).
5b. Add serum 100 μl/well serum samples diluted in PBS (recommended dilutions: 1:200-1:1600) and incubate the plates 2 hr at room temperature.
6b. Repeat washing (step 2).
7b. Add 100 μl/well anti-mouse-IgG1-HRP or anti-mouse-IgG2a-HRP diluted in PBS (dilution: 1:2000) and incubate 1 hr at room temperature.
8b. Repeat washing (step 2).
9b. Develop the reactions:
- For anti-mPG-IgG1 : Mix 20 mg OPD in 60 ml PBS, add 10 μl concentrated (30%) H2O2, and pipet 100 μl/well.
- For anti-mPG-IgG2a : Add 100 μl/well TMB substrate (freshly mixed: 50%-50% of BD OptEIA™ A and B substrate components).
10b. Stop the color reaction with 25 μl of 4 M H2SO4 or HCl, and read the plates with automatic ELISA reader as follows:
- Anti-mPG-IgG1 (with OPD): at 490 nm.
- Anti-mPG-IgG2a (with TMB): at 450 nm.
11b. Use the optical density values for statistical analysis.
Measurement of Cytokine Levels in Serum Samples
The most commonly used techniques for measuring cytokines are sandwich ELISA assays and the BD Cytometric Bead Array (CBA) technology based on flow cytometry. Both offer the advantages of sufficient sensitivity and accuracy, but each also has disadvantages and additional advantages. After careful consideration, either method is suitable for determining the cytokine content of serum and supernatants. Because cytokine concentrations are typically very low (in the picomolar range) in both serum and supernatant samples, the sandwich ELISA technique necessitates direct pipetting of samples onto plates without dilution. Given that an average of 200-300 μl of serum can be collected from a mouse, a maximum of two different cytokines can be measured simultaneously with ELISA assays. In such cases, serum must be collected again and transferred to subsequent ELISA plates to enable measurement of individual cytokines, either staggered in time or on different days. Approximately 500 μl of supernatant from spleen cell cultures (due to loss by evaporation during culture) can be utilized for measurements, allowing the simultaneous measurement of five different cytokines. When dealing with multiple cytokines, the solution is to re-collect and transfer the supernatant after incubation to additional cytokine ELISA plates. The challenges stemming from limited sample volumes described above can be mitigated by employing the CBA technique. Although using CBA may be more expensive than ELISA, it offers the advantage of enabling the measurement of numerous cytokines simultaneously using only a small volume of samples. This capability allows both sample and time savings.
Materials
- Sera of BALB/c mice with GIA (Basic Protocol, step 9)
- For sandwich ELISA assay:
- Sandwich ELISA DuoSet (R&D Systems) for mouse IL-1β, IL-4, IL-6, IL-17, IL-23, IFNγ, and TNFα
- Nunc Maxisorp 96-well ELISA plates
- Phosphate-buffered saline (PBS)
- Washing buffer: 0.05% (v/v) Tween-20 in PBS
- Blocking buffer: 1% (v/v) BSA in PBS
- 3,3′,5,5′-Tetramethylbenzidine (TMB; BD OptEIA™ TMB Substrate Reagent Set, BD OptEIA™ TMB Substrate Reagent Set, cat. no. 555214)
- 4 M H2SO4 or HCl
- Automatic ELISA microplate reader
- For CBA assay:
- 96-well U-bottom sample plate (BD, Falcon)
- BD™ Cytometric Bead Array (CBA) Mouse kit (with desired cytokine-specific beads)
- Flow cytometer with HTS module
Cytokine measurement by sandwich ELISA assay
The following brief description is based on the instructions from the ELISA kit manufacturer (R&D Systems).
1c. Coat Nunc Maxisorp 96-well ELISA plates with the capture antibodies diluted in PBS (working concentration: 4 µg/ml) overnight in humidified atmosphere (sealed aluminum foil with wet paper towel) on room temperature.
2c. Wash three times with 400 µl/well of washing buffer (0.05% Tween-20 in PBS) and then block the nonspecific protein binding sites with 400 µl/well blocking buffer (1% BSA in PBS) for 1 hr.
3c. After washing, pipet the standard samples of known concentrations (serial dilutions from 1000 pg/ml to 15.6 pg/ml of 1% BSA in PBS) and the unknown samples into the appropriate plate wells, and incubate the plates for 2 hr at room temperature.
4c. Wash the plates: Add biotinylated detection antibodies (working concentration: 200 ng/ml) diluted in 1% BSA in PBS, and incubate plates for 2 hr at room temperature.
5c. Dilute the streptavidin-HRP (provided in liquid form as part of the kit) 40-fold in blocking buffer, pipet 100 µl per well into the plates, and incubate plates for 20 min at room temperature.
6c. Add 100 µl TMB per well to develop the reaction, stop the color reaction with 25 µl per well of 4 M H2SO4 or HCl, and measure the optical density using ELISA reader.
7c. Generate a standard curve based on the serial diluted standard samples.
Cytokine measurement with CBA technology
1d. Incubate 50 µl of capture bead mixture with 50 µl supernatant/serum/standard diluted 1:50 on a 96-well U-bottom sample plate.
2d. Shake the plate for 5 min and then incubate at room temperature in the dark for 1 hr.
3d. Add 50 μl of phycoerythrin (PE)-labeled detection antibody mixture (included in the kit) to each well. Shake the plates again for 5 min, followed by a 1-hr incubation in the same manner as before.
4d. Wash the samples twice with CBA washing buffer and then resuspend in 150 μl washing buffer. Measure the samples on a flow cytometer using the HTS module.
Support Protocol 6: MEASUREMENT OF rhG1-INDUCED PROLIFERATION AND CYTOKINE PRODUCTION IN SPLEEN CELL CULTURE
In addition to serum, spleens can also be isolated from mice with GIA. Spleens isolated under sterile conditions can be used to set up cell cultures for use in determining the proliferation and cytokine (IL-1β, IL-4, IL-6, IL-17, IL-23, IFNγ, TNFα) production of spleen cells upon antigen stimulus (Table 3). Proliferation and cytokine levels correlate with arthritis severity, and thus these measurements provide valuable additional information alongside severity scores (Support Protocol 4) and serum parameters (Support Protocol 5).
| Cytokinea | Mean | SEM |
|---|---|---|
| IL-4 | 978.2 | 217.8 |
| IL-6 | 423.2 | 127.5 |
| IL-17 | 330.4 | 165.7 |
| IFNγ | 1765.8 | 593.6 |
| TNFα | 352.2 | 51.8 |
-
Spleen cells were stimulated with rhG1 for 5 days. Mean and SEM values were calculated from the data of 9 mice.
- a
Presented as concentration values (pg/ml).
Materials
-
BALB/c mice with GIA (Basic Protocol, step 10)
-
DMEM cell culture medium, sterile
-
Hemolysis buffer
-
Fetal bovine serum (FBS), sterile
-
Promega CellTiter96® Nonradioactive Cell Proliferation Assay
-
Forceps and scissors, sterile
-
15-ml conical centrifuge tube with cap, sterile
-
Petri dishes, sterile
-
Stainless-steel mesh: Spectrum™ Spectra Mesh™ Woven Filters
-
96-well flat-bottom cell culture plates, sterile
Preparation of spleen cell cultures
1.Isolate the spleens in sterile 15-ml conical tubes with caps filled with 5 ml each of sterile DMEM. Transfer the spleens and DMEM into sterile petri dishes, and gently disassociate the spleens by mashing them through sterile stainless steel mesh. Resuspend and transfer the samples back into the 15-ml centrifuge tubes, add 10 ml hemolysis buffer to each tube, tighten the cap, and gently rotate the manually until hemolysis is complete. Then, centrifuge the samples for 5 min at 183 rcf.
2.Discard the supernatant and resuspend the pellet in 5 ml DMEM supplemented with 10% FBS. Count the cells and adjust the concentration to yield a cell number of 12 × 106 cells in 2 ml DMEM with 10% FBS.
Measurement of rhG1-induced proliferation in spleen cell culture
3a. Culture 3 × 105 cells/well with 1.5 μg rhG1 or without rhG1 antigen in triplicate on 96-well plates for 5 days. Proliferation rates can be assessed using the Promega CellTiter96® Nonradioactive Cell Proliferation Assay according to the manufacturer's instructions.
Measurement of cytokine levels in supernatant of spleen cell cultures
3b. Seed 1.8 × 106 cells/in 600 μl medium/well onto 48-well plates with 1.5 μg of rhG1 or without rhG1 antigen. Culture for 5 days in a 37°C, 5% CO2 incubator, and then, collect the supernatants and store at −20°C until cytokine measurements are performed.
Support Protocol 7: HISTOLOGICAL ASSESSMENT OF ARTHRITIC LIMBS
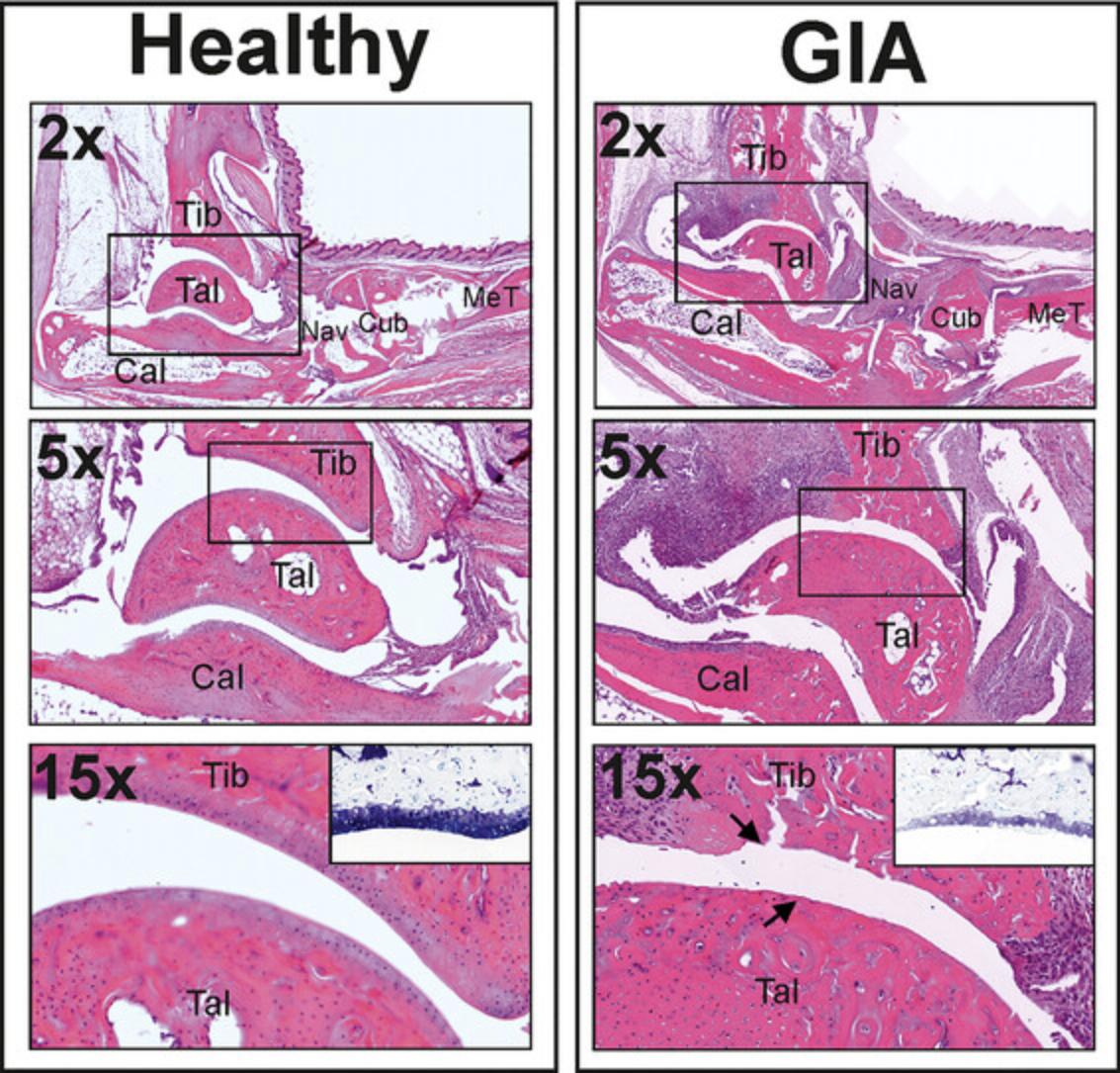
In addition to the laboratory parameters above (see Support Protocols 5 and 6), the degree of inflammation can also be effectively characterized using histological techniques, by assessing cellular infiltration around the joints, cartilage, and bone destruction that is characteristic of GIA (Fig. 5).
Materials
-
BALB/c mice with GIA (Basic Protocol, step 11)
-
10% (v/v) paraformaldehyde in PBS
-
10% (w/v) EDTA
-
Mayer's hematoxylin and eosin (HE) solution or toluidine blue
-
Forceps and scissors
-
50-ml conical centrifuge tube with cap
-
Additional reagents and equipment for paraffin embedding and sectioning (Zeller, 2001)
1.Collect the hind legs of arthritic mice after euthanasia (Basic Protocol, step 11) and fix in 10% paraformaldehyde.
2.Decalcify the specimens with 10% EDTA at 37°C for 1 day.
3.Embed specimens in paraffin and prepare 4-µm-thick sections.
4.Stain the sections with the selected staining solution—e.g., hematoxylin and eosin (HE) solution or toluidine blue—using an automated staining system (e.g., Leica ST 4040 linear automatic stainer; Leica Biosystems, Germany).
5.Finally, scan the slides using an appropriate tissue section scanning system (e.g., Pannoramic MIDI Scanner; 3DHistech, Hungary).
6.Images can be analyzed using an appropriate software (e.g., Pannoramic View Software; 3DHistech, Hungary).
Support Protocol 8: EVALUATION OF ARTHRITIS WITH MICRO-COMPUTED TOMOGRAPHY
An advantage of CT imaging is its ability to detect subtle changes in bone structure that may not be apparent using other imaging techniques (Fig. 6). This can be particularly useful for early diagnosis and monitoring of arthritis progression. Nowadays, high-resolution small-animal micro-CT machines are already available, providing another potential method for assessing the severity of arthritis in mice. The micro-architectural changes in the ankle and proximal foot joints can be easily visualized, and a 3D scan can be reconstructed using CT analysis software (Fig. 6). In addition to providing a representative display of bone adhesions and erosion, the method is also suitable for assessing the mean bone density. This additional information is crucial for understanding the extent of joint inflammation. A further advantage this technique is that it can be repeated during the induction period of GIA to follow the progression of the disease.
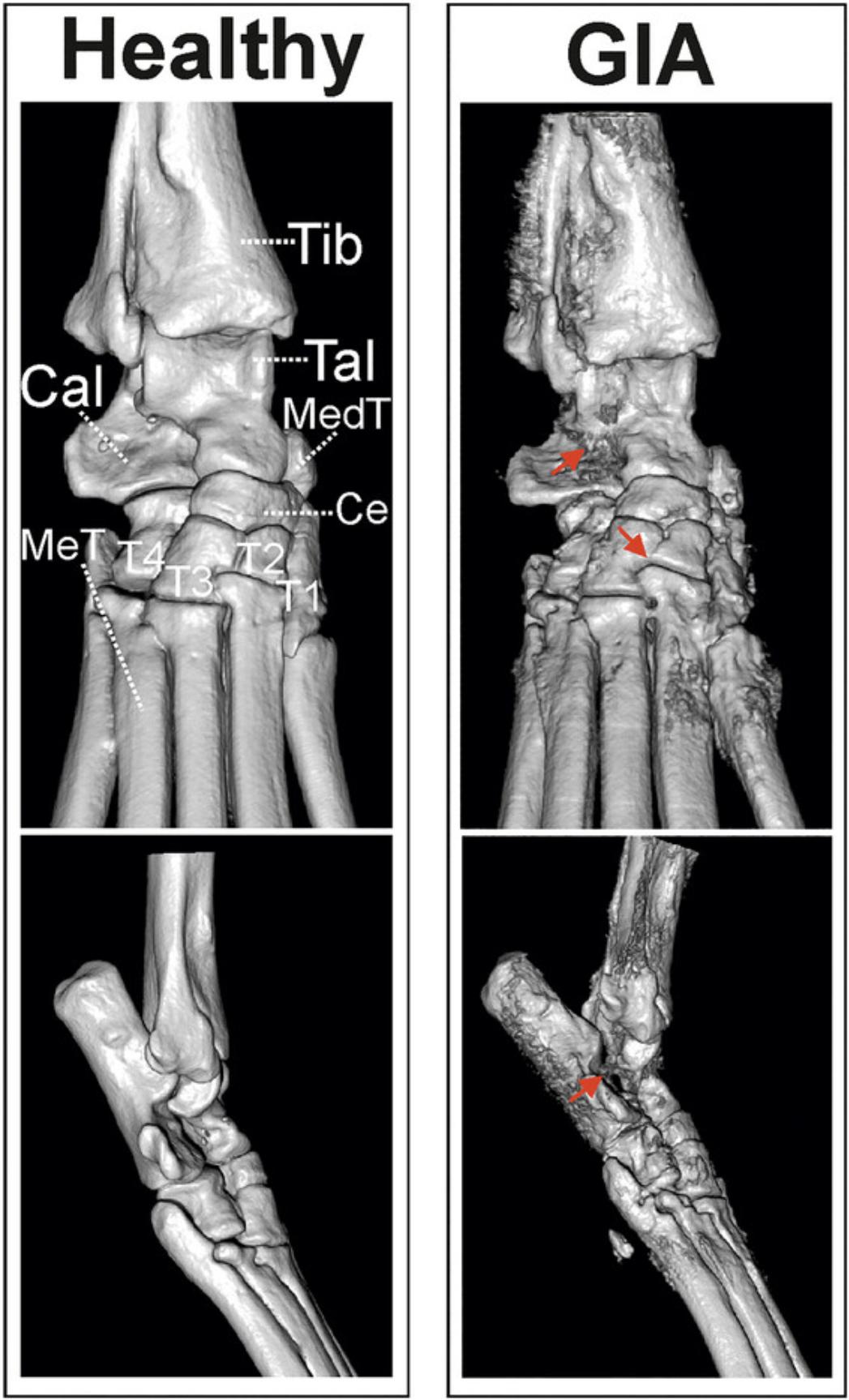
Materials
-
BALB/c mice with GIA (Basic Protocol)
-
Ketamine and xylazine, for anesthesia
-
In vivo micro-CT system: e.g., Skyscan 1176 in vivo micro-CT System
-
CT Analyzer software
1.Anesthetize mice with 120 mg/kg ketamine and 6 mg/kg xylazine, delivered intraperitoneally following standard laboratory practice, before beginning measurement.
2.Use the Skyscan 1176 in vivo micro-CT system to scan the right hind paws of the mice. A 0.5-mm A1 filter is recommended, with a voxel size of 17.5 µm and a 50-kV tube voltage; the tube current should be fixed at 500 µA.
3.Use CT Analyzer software to reconstruct the 3D scans.
COMMENTARY
Background Information
PG aggrecan
Proteoglycans (PGs) are complex macromolecules composed of a core protein to which glycosaminoglycan (GAG) and N- and O-linked oligosaccharide side chains are attached (Fig. 1; Hanyecz et al., 2014). The PG aggrecan, comprising 10%-20% of the wet weight, provides compressive strength to the articular cartilage. There are two major classes of PGs in articular cartilage: large aggregating PG monomers or aggrecans (henceforth PG aggrecan) and small PGs including decorin, biglycan, and fibromodulin (Cs-Szabó et al., 1995; Roughley, 2006). The core protein of PG aggrecan contains three globular domains: G1 and G2 near the N-terminus and G3 at the C-terminus (Fig. 1). The G1 and G2 domains are separated by a short interglobular domain (IGD), and the G2 and G3 domains are separated by a long GAG-attachment region to which hundreds of keratan sulfate (KS) and chondroitin sulfate (CS) side chains attach (Fig. 1). Importantly, T cell responses to four epitopes were clearly associated with arthritis induction in mice (“arthritogenic” epitopes; Buzás et al., 2005). Of these four epitopes, three are found in the G1 and only one in the G3 domain of PG aggrecan (Fig. 1), underlining the importance of these regions in the induction of arthritis (Buzás et al., 2005).
PG aggrecan-induced arthritis (PGIA) model
The direct predecessor of the GIA model was proteoglycan-induced arthritis (PGIA; Glant et al., 1987). Repeated intraperitoneal immunization of BALB/c mice with human cartilage PG aggrecan led to severe progressive polyarthritis representing many aspects of human RA (autoantibodies were produced and T cell activation was observed, together with similar histopathological and radiological characteristics; Mikecz et al., 1987). The immunological background of the disease was a cross-reaction to mouse PG that was induced by the human PG aggrecan (Glant et al., 2003). Several mouse strains were tested for PGIA susceptibility (Mikecz et al., 1987), but experiments showed that only the BALB/c strain is susceptible. Interestingly, different BALB/c colonies showed some variation in arthritis susceptibility, which could be due to some genetic shift among separately bred colonies (Farkas et al., 2009). Only one study showed that PGIA could be induced outside the BALB/c strain, in the C3H/HeJCr substrain, but all other C3H mice tested showed resistance (Glant et al., 2001). Genetic studies indicated that this susceptibility is based on MHC (I-Ad) and non-MHC genes. The age of mice is also critical for PGIA, with the best results being obtained when using 5- to 6-month-old mice (Tarjanyi et al., 2009). Originally, immunization was done in combination with Freund's adjuvant (Glant et al., 1987), but this was later replaced by DDA (Hanyecz et al., 2004). In earlier studies of PGIA, the dominance of Th1 cells was observed (Finnegan et al., 1999; Holló et al., 2000); however, later an additional role of Th17 cells was established (Boldizsar et al., 2009; Doodes et al., 2010). Moreover, a T cell receptor (TcR)-transgenic mouse strain was also generated wherein the T cells recognized an immunodominant epitope located in the G1 domain of human PG aggrecan (Berlo et al., 2006). This PG-specific TcR-transgenic strain is extremely sensitive to PGIA, with the disease developing in most mice after only two immunizations (instead of the usual three; Berlo et al., 2006). Because the receptor specificity of the T cells isolated from PG-specific TcR transgenic is known, this model is particularly useful for studying antigen-specific T cell activation and apoptosis in autoimmune arthritis (Olasz et al., 2012; A. Hanyecz et al., 2014).
rhG1-induced arthritis (GIA) model
A major limitation of the PGIA model was that the antigen was prepared from human cartilage and thus sources were limited. To overcome this problem, a genetic construct containing the G1 domain of the human PG aggrecan in fusion with a heavy chain of the mouse IgG2a was generated and introduced into CHO cells (Murad et al., 2005). Culturing these cells produced large amounts of rhG1 that could be purified easily on protein G columns, similar to monoclonal antibodies (Murad et al., 2005). This method provided an unlimited antigen source. Comparison of GIA with the predecessor PGIA model showed that both cause a similar clinical and histopathological picture, T and B cell activation, and inflammatory cytokine production (Glant et al., 2011). In GIA, significantly greater IL-17 and IFNγ production by the spleen cells of arthritic mice is observed, together with higher levels of RF and lower levels of anti-CCP in the serum, as compared to PGIA (Glant et al., 2011). GIA has successfully used in recent years to study the roles of T cell activation and apoptosis (Kugyelka et al., 2019) and different aspects of the spleen's role in autoimmune arthritis (Khanfar et al., 2020; Khanfar et al., 2022; Khanfar et al., 2024).
Critical Parameters
The quality of the antigen and immunization of 5- to 6-month-old BALB/c mice are critical to induction of GIA.
Production and purification of rhG1
Culturing protocol for the recombinant antigen-producing cell line must be optimal to achieve good yields of the antigen. For optimal results, a 70%-80% confluent monolayer of CHO cells must be maintained throughout cell culture. Clumping of the cells after passage should be avoided. During harvesting and addition of fresh medium, care must be to avoid disrupting monolayer of CHO cells, which can decrease the antigen yield. The purification with protein G must also be optimized to maximize yield. The manufacturer's instructions must be taken into consideration during the affinity chromatography.
Preparation of the DDA adjuvant
If DDA preparation is done correctly, a thick gel will form after the repeated boiling and cooling of the DDA. The recombinant antigen and the sterile PBS must be added to the DDA and thoroughly mixed by pipetting up and down several times to achieve a homogenous emulsion. Care should be taken to minimize foam formation by avoiding excessive formation of air bubbles during this process.
Mice
The best results are obtained with female 5- to 6-month-old BALB/c mice. The use of younger mice may lead to weaker arthritis. GIA can also be induced in male mice, but their aggressive behavior may hinder the experiments and make it impossible to objectively score arthritis. At the end of the experiment, when severe arthritis develops, food can be placed on the bottom of the cages for easier access.
Troubleshooting
Table 4 lists some potential problems that can occur when setting up the GIA system to study autoimmune arthritis, along with their possible causes and solutions.
| Problem | Possible cause | Solution |
|---|---|---|
| Low rhG1 yield | Suboptimal CHO cell numbers | Check the CHO cell confluence. |
| Suboptimal cell culture condition(s) | Check the medium, incubator, etc. | |
| Low arthritis incidence | Problem with the antigen | Check the used rhG1 on gel/western blot. |
| Problem with the adjuvant | Check that the DDA emulsion is prepared the right way; in the end, it should be a thick gel. | |
| Problem with immunization | Check the intraperitoneal injection. | |
| Problem with mice | Check that appropriate BALB/c mice are selected, paying attention to the age: in mice under 3 months of age, GIA induction is not optimal, so use 5- to 6-month-old mice. | |
| Low proliferation response | Problem with in vitro spleen cell culture | Check the cell numbers, antigen concentration, and culture conditions. |
| Low cytokine concentrations | Problem with in vitro spleen cell culture | Check the cell numbers, antigen concentration, and culture conditions. |
| Too intense or weak a signal on antibody ELISA | Problem with the dilution of sera | Decrease or increase sample dilution. |
Understanding Results
Clinical picture of arthritis
The first clinical signs of GIA usually develop 10-14 days after the second immunization (Fig. 4). During assessment, the investigator should evaluate the severity of inflammation in all four limbs. Typically scoring is done three times a week. Additionally, edema (swelling) of the paws can be characterized by measuring paw thickness using a digital caliper. Arthritis severity can be expressed as the cumulative score for the four limbs (0-16; Fig. 4) or as the thickness of hind or front limbs or ankles (typically between 2-4 mm; Fig. 4). Incidence values are usually provided as a percentage, which is calculated by dividing the number of arthritic mice by the total number of immunized mice in the experiment. Both the severity scores and the incidence are typically plotted over time (Fig. 4). The reliable and comparable clinical assessment of GIA requires practice and solid experience. It is also advisable to have the mice scored in parallel by more than one researcher independently.
Laboratory parameters
In case of the serum antibody parameters, ELISA results can be expressed as OD values or concentrations. We usually determine two isotypes of antibody for each specific antigen—IgG1 and IgG2a—because in mice these two antibody isotypes are associated with the Th2- and Th1-type immune responses (Finnegan et al., 1999; Holló et al., 2000; Boldizsar et al., 2009). Calculating the IgG2a to IgG1 ratio may be also useful indicator of the shift toward Th1 in GIA. In the case of the rhG1- and mouse-PG-specific antibodies, the use of a standard serum with known concentrations is helpful (standard sample dilutions from 1:3200 to 1:16,000 in PBS). Usual dilutions used for measurement of rhG1-specific antibody levels with indirect ELISA are as follows: IgG1, 1:4000 to 1:16,000; IgG2a, 1:3200 to 1:8000. Usual dilutions (in PBS) used for measurement of serum autoantibody levels (anti-mouse PG) with indirect ELISA are 1:200 to 1:1600. In the case of commercial ELISA kits to measure cytokines, RF, and anti-CCP concentrations, factory standards are provided. For the proliferation test, the results are usually expressed as the “stimulation index” (SI), which is calculated by dividing the O.D. values of the antigen-stimulated samples with the OD values of unstimulated samples from the corresponding mouse.
Production of rhG1
Production of rhG1 is critical to induce GIA. Typical yields are between 5-10 mg of recombinant protein per liter of CHO medium supernatant. After the purification, the antigen can be checked using SDS-PAGE or western blotting (Fig. 2). For certain purposes (e.g., antibody ELISA), enzymatic cleavage of the construct with Factor Xa may be necessary. In that case the result of the cleavage can also be assessed by SDS-PAGE or western blotting.
Time Considerations
- 1.Production of rhG1-Xa-mFc2a fusion protein with CHOK1 mammalian expression system: 3 weeks
- 2.Purification of the rhG1-Xa-mFc2a fusion protein by affinity chromatography: 1 week
- 3.Arthritis induction with repeated intraperitoneal immunization with the emulsion of rhG1-Xa-mFc2a and DDA adjuvant: antigen preparation, 1 hr; immunization, 2 min/mouse
- 4.Clinical assessment of arthritis: clinical scoring: 1 min/mouse; limb thickness measurement with digital caliper: 2 min/mouse
- 5.Micro-CT: 2 hr/mouse
- 6.Cleavage of rhG1-Xa-mFc2a with Factor Xa and purification of the G1 fragment: 1 day
- 7.Euthanasia of mice at the end of the experiment: 4-8 hr (strongly dependent on the number of mice)
- 8.Serum collection from blood samples with centrifugation: 1-2 hr (strongly dependent on the number of mice)
- 9.Spleen cell culture proliferation test: 5 days of incubation with antigen and 5 hr for non-radioactive assay
- 10.Measurement of cytokine levels from spleen cell cultures: 5 days of incubation with antigen in 48 well plates; cytokine ELISA: 24 hr (with overnight incubation); cytokine measurement with CBA technology: 4 hr
- 11.Serum anti-rhG1 antibody ELISA: 23 hr (with overnight incubation)
- 12.Serum anti-mPG antibody ELISA: 22 hr (with overnight incubation)
Acknowledgments
We would like to express our utmost gratitude to Prof. Tibor T. Glant and Prof. Katalin Mikecz (both formerly at Rush University Medical Center, Chicago, USA) for introducing us to the PGIA model and involving us in the testing of the GIA system while working in their laboratory between 2006 and 2008.Afterwards, upon returning to the University of Pécs, Hungary, we could not have established the GIA model successfully without their useful help and advice. Project no. TKP2021-EGA-10 has been implemented with support provided from the National Research, Development and Innovation Fund of Hungary, financed under the TKP2021-EGA funding scheme.
Author Contributions
Katalin Olasz : Investigation; methodology; writing—original draft. Ferenc Boldizsar : Conceptualization; funding acquisition; investigation; supervision; visualization; writing—review and editing.
Conflict of Interest
The authors have no conflict of interest.
Open Research
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Literature Cited
- Berlo, S. E., Guichelaar, T., Ten Brink, C. B., Van Kooten, P. J., Hauet-Broeren, F., Ludanyi, K., van Eden, W., Broeren, C. P., & Glant, T. T. (2006). Increased arthritis susceptibility in cartilage proteoglycan-specific T cell receptor-transgenic mice. Arthritis and Rheumatism , 54(8), 2423–2433. https://doi.org/10.1002/art.22013
- Boldizsar, F., Tarjanyi, O., Nemeth, P., Mikecz, K., & Glant, T. T. (2009). Th1/Th17 polarization and acquisition of an arthritogenic phenotype in arthritis-susceptible BALB/c, but not in MHC-matched, arthritis-resistant DBA/2 mice. International Immunology , 21(5), 511–522. https://doi.org/10.1093/intimm/dxp018
- Buzás, E. I., Végvári, A., Murad, Y. M., Finnegan, A., Mikecz, K., & Glant, T. T. (2005). T-cell recognition of differentially tolerated epitopes of cartilage proteoglycan aggrecan in arthritis. Cellular Immunology , 235(2), 98–108. https://doi.org/10.1016/j.cellimm.2004.08.006
- Cs-Szabó, G., Roughley, P. J., Plaas, A. H., & Glant, T. T. (1995). Large and small proteoglycans of osteoarthritic and rheumatoid articular cartilage. Arthritis and Rheumatism , 38(5), 660–668. https://doi.org/10.1002/art.1780380514
- Doodes, P. D., Cao, Y., Hamel, K. M., Wang, Y., Rodeghero, R. L., Mikecz, K., Glant, T. T., Iwakura, Y., & Finnegan, A. (2010). IFN-γ regulates the requirement for IL-17 in proteoglycan-induced arthritis. The Journal of Immunology , 184(3), 1552–1559. https://doi.org/10.4049/jimmunol.0902907
- Farkas, B., Boldizsar, F., Tarjanyi, O., Laszlo, A., Lin, S. M., Hutas, G., Tryniszewska, B., Mangold, A., Nagyeri, G., Rosenzweig, H. L., Finnegan, A., Mikecz, K., & Glant, T. T. (2009). BALB/c mice genetically susceptible to proteoglycan-induced arthritis and spondylitis show colony-dependent differences in disease penetrance. Arthritis Research & Therapy, 11(1), R21. https://doi.org/10.1186/ar2613
- Finnegan, A., Mikecz, K., Tao, P., & Glant, T. T. (1999). Proteoglycan (aggrecan)-induced arthritis in BALB/c mice is a Th1-type disease regulated by Th2 cytokines. Journal of Immunology (Baltimore, Md. : 1950) , 163(10), 5383–5390.
- Glant, T. T., Mikecz, K., Arzoumanian, A., & Poole, A. R. (1987). Proteoglycan-induced arthritis in BALB/c mice. Clinical features and histopathology. Arthritis and Rheumatism , 30(2), 201–212. https://doi.org/10.1002/art.1780300211
- Glant, T. T., Brdos, T., Chandrasekaran, C. V. R., Valdz, J. C., Otto, J. M., Gerard, D., Velins, S., Lovász, G., Zhang, J., Mikecz, K., & Finnegan, A. (2001). Variations in susceptibility to proteoglycan-induced arthritis and spondylitis among C3H substrains of mice: Evidence of genetically acquired resistance to autoimmune disease. Arthritis and Rheumatism , 44(3), 682–692. https://doi.org/10.1002/1529-0131(200103)44:3<682::AID-ANR118>3.0.CO;2-E
- Glant, T. T., Radacs, M., Nagyeri, G., Olasz, K., Laszlo, A., Boldizsar, F., Hegyi, A., Finnegan, A., & Mikecz, K. (2011). Proteoglycan-induced arthritis and recombinant human proteoglycan aggrecan G1 domain-induced arthritis in BALB/c mice resembling two subtypes of rheumatoid arthritis. Arthritis & Rheumatism, 63(5), 1312–1321. https://doi.org/10.1002/art.30261
- Glant, T. T., Finnegan, A., & Mikecz, K. (2003). Proteoglycan-induced arthritis: Immune regulation, cellular mechanisms, and genetics. Critical Reviews in Immunology , 23(3), 199–250. https://doi.org/10.1615/critrevimmunol.v23.i3.20
- Gordon, W. C., Prager, M. D., & Carroll, M. C. (1980). The enhancement of humoral and cellular immune responses by dimethyldioctadecylammonium bromide. Cellular Immunology , 49(2), 329–340. https://doi.org/10.1016/0008-8749(80)90034-9
- Hanyecz, A., Olasz, K., Tarjanyi, O., Nemeth, P., Mikecz, K., Glant, T. T., & Boldizsar, F. (2014). Proteoglycan aggrecan conducting T cell activation and apoptosis in a murine model of rheumatoid arthritis. BioMed Research International , 2014, https://doi.org/10.1155/2014/942148
- Hanyecz, A., Berlo, S. E., Szántó, S., Broeren, C. P. M., Mikecz, K., & Glant, T. T. (2004). Achievement of a synergistic adjuvant effect on arthritis induction by activation of innate immunity and forcing the immune response toward the Th1 phenotype. Arthritis & Rheumatism, 50(5), 1665–1676. https://doi.org/10.1002/art.20180
- Hilgers, L. A., & Snippe, H. (1992). DDA as an immunological adjuvant. Research in Immunology , 143(5), 494–496. https://doi.org/10.1016/0923-2494(92)80060-x
- Holló, K., Glant, T. T., Garzó, M., Finnegan, a., Mikecz, K., & Buzás, E. (2000). Complex pattern of Th1 and Th2 activation with a preferential increase of autoreactive Th1 cells in BALB/c mice with proteoglycan (aggrecan)-induced arthritis. Clinical and Experimental Immunology , 120(1), 167–173. https://doi.org/10.1046/j.1365-2249.2000.01174.x
- Katz, D., Lehrer, S., Galan, O., Lachmi, B., Cohen, S., Inbar, I., Samina, I., Peleg, B., Heller, D., Yadin, H., Chai, D., Freeman, E., Schupper, H., & Fuchs, P. (1996). Unique immunomodulating properties of dimethyl dioctadecyl ammonium bromide (DDA) in experimental viral vaccines. Advances in Experimental Medicine and Biology , 397, 115–125. https://doi.org/10.1007/978-1-4899-1382-1_16
- Katz, D., Lehrer, S., Galan, O., Lachmi, B. E., & Cohen, S. (1991). Adjuvant effects of dimethyl dioctadecyl ammonium bromide, complete Freund's adjuvant and aluminium hydroxide on neutralizing antibody, antibody-isotype and delayed-type hypersensitivity responses to Semliki forest virus in mice. FEMS Microbiology Immunology , 3(6), 305–320. https://doi.org/10.1111/j.1574-6968.1991.tb04255.x
- Khanfar, E., Olasz, K., Fanni, G., Botz, B., Gábris, F., Gajdócsi, E., Botz, B., Kiss, T., Kugyelka, R., Berki, T., Balogh, P., & Boldizsár, F. (2020). Ameliorated autoimmune arthritis and impaired B cell receptor-mediated Ca2+ influx in Nkx2-3 knock-out mice. International Journal of Molecular Sciences , 21(17), 1–16. https://doi.org/10.3390/ijms21176162
- Khanfar, E., Olasz, K., Gajdócsi, E., Jia, X., Berki, T., Balogh, P., & Boldizsár, F. (2022). Splenectomy modulates the immune response but does not prevent joint inflammation in a mouse model of RA. Clinical and Experimental Immunology , 209(2), 201–214. https://doi.org/10.1093/cei/uxac052
- Khanfar, E., Olasz, K., Gál, S., Gajdócsi, E., Kajtár, B., Kiss, T., Balogh, P., Berki, T., & Boldizsár, F. (2024). Splenectomy at early stage of autoimmune arthritis delayed inflammatory response and reduced joint deterioration in mice. Clinical and Experimental Immunology , uxae013. https://doi.org/10.1093/cei/uxae013
- Kugyelka, R., Prenek, L., Olasz, K., Kohl, Z., Botz, B., Glant, T. T., Berki, T., & Boldizsár, F. (2019). ZAP-70 regulates autoimmune arthritis via alterations in T cell activation and apoptosis. Cells , 8(5), 504. https://doi.org/10.3390/cells8050504
- Mikecz, K., Glant, T. T., & Poole, A. R. (1987). Immunity to cartilage proteoglycans in BALB/c mice with progressive polyarthritis and ankylosing spondylitis induced by injection of human cartilage proteoglycan. Arthritis and Rheumatism , 30, 306–318. https://doi.org/10.1002/art.1780300310
- Murad, Y. M., Szabó, Z., Ludányi, K., & Glant, T. T. (2005). Molecular manipulation with the arthritogenic epitopes of the G1 domain of human cartilage proteoglycan aggrecan. Clinical and Experimental Immunology , 142(2), 303–311. https://doi.org/10.1111/j.1365-2249.2005.02921.x
- Olasz, K., Boldizsar, F., Kis-Toth, K., Tarjanyi, O., Hegyi, A., van Eden, W., Rauch, T. A., Mikecz, K., & Glant, T. T. (2012). T cell receptor (TCR) signal strength controls arthritis severity in proteoglycan-specific TCR transgenic mice. Clinical & Experimental Immunology, 167(2), 346–355. https://doi.org/10.1111/j.1365-2249.2011.04506.x
- Roughley, P. J. (2006). The structure and function of cartilage proteoglycans. European Cells & Materials, 12, 92–101. https://doi.org/10.22203/ecm.v012a11
- Tarjanyi, O., Boldizsar, F., Nemeth, P., Mikecz, K., & Glant, T. T. (2009). Age-related changes in arthritis susceptibility and severity in a murine model of rheumatoid arthritis. Immunity & Ageing, 6(1), 8. https://doi.org/10.1186/1742-4933-6-8
- Zeller, R. (2001). Fixation, embedding, and sectioning of tissues, embryos, and single cells. Current Protocols in Pharmacology , 7, A.3D.1–A.3D.9. https://doi.org/10.1002/0471141755.pha03ds07

