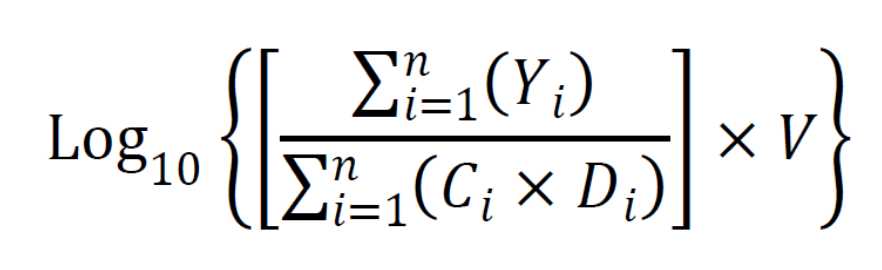Quantitative Method to Measure Disinfectant Product Efficacy
John Hilgren
Disclaimer
None
Abstract
This protocol is used for measuring the inactivation of Pseudomonas aeruginosa by liquid disinfectants. In brief, stainless-steel discs are inoculated with single 10 µL drops of a P. aeruginosa suspension also containing a three-part soil mixture. The three-part soil consists of bovine mucin, bovine serum albumin and yeast extract. The inoculum drops are dried on the discs and then the discs are transferred into flat-bottom vials for testing. Testing involves overlaying each dried inoculum spot with 50 µL of a disinfectant working solution or a negative control solution. A contact time is allowed to elapse and then a disinfectant-neutralizing agent is added. Any material still adsorbed on the carrier is eluted by vigorous washing/mixing of the carrier in the neutralizer. The neutralizer eluate and 10-fold serial dilutions of the eluate are filtered through 0.2-micron membrane filters. The filters are transferred to the surfaces of agar plates and incubated. Colony forming units on the filters are counted and log reductions for the disinfectant treatments are calculated.
Steps
Calculate log reductions
Prepare vials of frozen stock culture
Prepare vials of frozen stock culture
Rehydrate a lyophilized culture of Pseudomonas aeruginosa ATCC 15442 with 5 to 6 mL of Tryptic Soy Broth (TSB)
Re-suspend the growth in the cryoprotectant solution using a sterile spreader without damaging the agar surface.
Aspirate the suspension from the plate with a pipette and transfer it into a sterile vessel.
Repeat the growth harvesting procedure with the remaining plates and continue adding the subsequent suspensions to the same collection vessel.
Mix the vessel contents and dispense 0.5-1.0 mL aliquots of the harvested suspension into cryovials for freezing.
Confirm the identity and purity of the culture by culture characteristics, biochemical characteristics (e.g., Gram-negative, Oxidase positive), fermentation, enzymatic reactions and/or molecular techniques.
Store the cryovials at -70°C or lower for a maximum of 18 months.
Mix thoroughly.
Incubate broth culture at 36°C ± 1°C for 24 h ±2 h.
Streak a loopful of the broth culture onto two Tryptic Soy Agar (TSA) plates to obtain isolated colonies.
Incubate TSA plates for 24 h ± 2 h at 36°C ± 1°C.
From the TSA plates, select 3-5 isolated colonies and re-suspend in 1 mL of TSB. Select colonies from each of the two phenotypes present.
Spread plate 0.1 mL of the TSB suspension onto each of 6-10 TSA plates.
Incubate the TSA plates for 24 h ± 2 h at 36°C ± 1°C.
Add approximately 5 mL of sterile cryoprotectant solution (TSB with 15% (v/v) glycerol) to the surface of each TSA plate.
Prepare the test culture (minus the soil load)
Prepare the test culture (minus the soil load)
Prepare tubes of Synthetic Broth (SB) by adding 0.1 mL of 10% sterile dextrose (w/v) solution to each 10 mL tube of SB and mix.
Re-suspend the pellet in 5 mL to 10 mL Phosphate Buffered Saline (PBS).
Create a homogenous mixture by vortex-mixing. Tap the centrifuge tube briskly if parts of the pellicle remain after mixing – this will assist in disaggregating the pellet. If tapping is necessary, then follow tapping with more vortex-mixing until a visibly homogenous suspension is achieved.
Dilute the 5 mL to 10 mL of resuspended culture in additional PBS, as needed, to achieve a mean control carrier count level of 5 log ± 0.5 log CFU/carrier after drying. The culture suspension from this step is then used to prepare the final test suspension (containing the soil load).
Remove a cryovial of the stock culture from the freezer and allow it to thaw at room temperature.
Add 0.1 mL of thawed stock culture to 10 mL of SB + dextrose and mix.
Incubate the inoculated SB + dextrose under static conditions for 24 h ± 2 h at 36°C ± 1°C. The 24 h ± 2 h culture is expected to contain approximately 108 CFU/mL.
Confirm there is a visible pellicle on the surface of the culture. Discard the culture if the pellicle has been disrupted or there are pellicle fragments in the culture.
Remove the pellicle from the surface of the culture and around the sidewalls of the vessel with vacuum suction.
Using a serological pipette, withdraw the remaining broth culture (approximately 7 to 8 mL) avoiding any sediment on the bottom of the tube and transfer it into a 15 mL conical centrifuge tube.
Within 15 minutes of completing the previous step, centrifuge contents of the conical centrifuge tube at 5,000 × g for 20 minutes.
Remove and discard the supernatant without disrupting the pellet.
Prepare the final test suspension containing soil load
Prepare the final test suspension containing soil load
Combine the following three soil components together: 25 µL BSA stock, 35 µL yeast extract stock, 100 µL mucin stock.
Vortex the three-part soil suspension for 10 seconds.
Add 340 µL of the test culture to the three-part soil, then mix.
Maintain the final test suspension at room temperature until it is used to inoculate carriers. Inoculate all carriers within 30 minutes of final test suspension preparation.
Inoculate and dry the carriers
Inoculate and dry the carriers.
Place carriers with the brushed side facing upward inside an empty, sterile plastic Petri dish. There should be no more than 20 carriers per dish. Inoculate three (3) carriers for each antimicrobial test condition and three (3) carriers to serve as control carriers. Inoculate additional carriers to serve as extras, in case they are needed (e.g., when a carrier is mishandled).
Using a positive displacement pipette with a 10 µL tip, withdraw 10 µL of the final test suspension and deposit it at the center of each carrier, keeping the pipette perpendicular to the carrier during deposition of the final test suspension. Avoid contact of pipette tip with carrier. Do not spread the final test suspension with the pipette tip. To maintain culture consistency, vortex-mix the final test suspension frequently during inoculation of the carriers. The same pipette tip may be used to inoculate all carriers. Discard inoculated carriers if the final test suspension runs over the edge of the carrier.
Transfer the Petri dish(es) with the inoculated carriers into a desiccation unit and remove the lid of the Petri dish. Do not exceed 40 inoculated carriers per desiccator.
Close the desiccation unit and apply vacuum.
Maintain a vacuum of 0.068 MPa to 0.085 MPa for the duration of the drying procedure.
Maintain the inoculated carriers in the desiccation unit (under vacuum) at room temperature for 45 to 60 minutes.
Visually inspect the inoculated carriers to verify that the inoculation spot is visibly dry. Do not use carriers that are visibly wet for testing. Do not use carriers if the inoculum has spread to near the edge of the carrier.
After drying and removal from the desiccation unit, hold carriers in a closed Petri dish at room temperature for up to 30 minutes before a test or control substance is applied. Do not use carriers for testing if they have been stored for longer than 30 minutes.
Expose dried inoculated carriers to the test substance or control substance
Expose dried inoculated carriers to the test substance (i.e., the disinfectant solution) or control substance. The control substance is PBS.
Use 3 carriers for each test condition, and 3 corresponding control carriers.
Using a sterile forceps, transfer each dried carrier with the inoculated side up to a flat-bottom vial and cap the vial. Repeat until all carriers are transferred.
In a timed fashion with appropriate intervals, sequentially deposit 50 µL of the antimicrobial test substance (at room temperature) over the dried inoculum on each test carrier, ensuring complete coverage. Apply the antimicrobial test substance with a pipet that is held vertically. Use a new pipette tip for each carrier; do not touch the carrier surface with the pipette tip during the application of the antimicrobial test substance or the control substance; replace with new carrier(s) and vial(s) if this occurs. Do not cap the vials. If the antimicrobial test substance or control substance runs off the carrier or does not completely cover the inoculum spot, then discard that carrier and replace with a new carrier. Repeat the same procedure for control carriers last, except use PBS instead of the antimicrobial test substance.
Neutralize the test substance
Neutralize the test substance
At the end of the timed contact period, add 10 mL of the neutralizer (Letheen broth with 0.5% Tween® 80 and 0.07% Lecithin) to each vial in the specified order according to the predetermined schedule.
Vortex-mix each vial for 2 to 3 seconds following the addition of the neutralizer.
Following the neutralization of the entire set of carriers, vortex-mix each vial for 30 seconds. Ensure that the liquid and carrier are both spinning in the vial during vortex-mix. Do not remove the carrier from the vial.
Dilute and plate the neutralized sample
Dilute and plate the neutralized sample.
Within 30 minutes of neutralization, dilute and filter all samples; prioritizing test substance treated carriers first.
Incubate all plates at 36°C ± 1°C. For control carriers, incubate for 48 ± 4 hours. For treatments, incubate for 72 ± 4 hours.
Transfer 1 mL of the neutralized sample into 9 mL PBS.
Prepare additional 10-fold serial dilutions as necessary to achieve countable filters (0-200 CFU).
Pre-wet the membrane filter (0.2 µm pore-size PES membrane) with 10 mL of PBS.
Add samples to filtering units; use a separate membrane filter for each sample. If a carrier falls onto the filter membrane, aseptically remove it using sterile forceps. If the filter membrane is accidentally punctured in the process of removing the carrier, then discard the membrane.
Rinse each empty sample vessel with 10 mL of PBS. Vortex-mix for 4 seconds and pour the vial contents into the same filter unit.
Rinse the interior sidewalls of the membrane filtering unit with 20 mL of PBS.
Appy a vacuum to the filter unit to pull the liquid through the filter.
With the vacuum on, aseptically remove the membrane filter and place it onto the surface of a TSA plate. Apply the filter to the agar surface in such a way to minimize the trapping of air bubbles between the filter and the agar surface.
Count colonies and record results
Count colonies and record results.
Count the colony forming units (CFUs) on each plated filter. If the number of colonies on a filter exceeds 200, then record as Too Numerous to Count (TNTC). If no colonies are present, then record as zero.
Verify that colony appearances on the plated filters are consistent with P. aeruginosa . Random contamination with non- P. aeruginosa CFUs that does not obscure counting of P. aeruginosa does not invalidate the test. Randomness of contamination can be confirmed using biochemical tests (e.g., Gram stain, catalase test, oxidase test). Consistent contamination across multiple plated filters indicates a systematic problem and the test should be repeated.
Calculate log densities
Calculate log densities
Where:
Y = CFU per filter,
C = volume filtered,
V = total volume of neutralizer,
D = 10-k,
k = dilution,
n = number of dilutions, and
i = lower limit of summation (the fewest number of dilutions).
When TNTC values are observed for each dilution filtered, substitute 200 for the TNTC at the highest (most dilute) dilution and account for the dilution factor in the calculations.
Calculate the mean log10 density (LD) for each set of three treatment conditions and the controls as follows:
Mean LD = [(log10CFU on carrier #1) + (log10CFU on carrier #2) + (log10CFU on carrier #3)]/3
Calculate log10 reductions
Calculate the log10 reduction (LR) for each treatment condition as follows:
LR = (Mean LD for Control Carriers) – (Mean LD for Treated Condition)
If no CFUs are recovered from each of the three treated carriers, then the log reduction is greater than or equal to the mean control carrier log density.