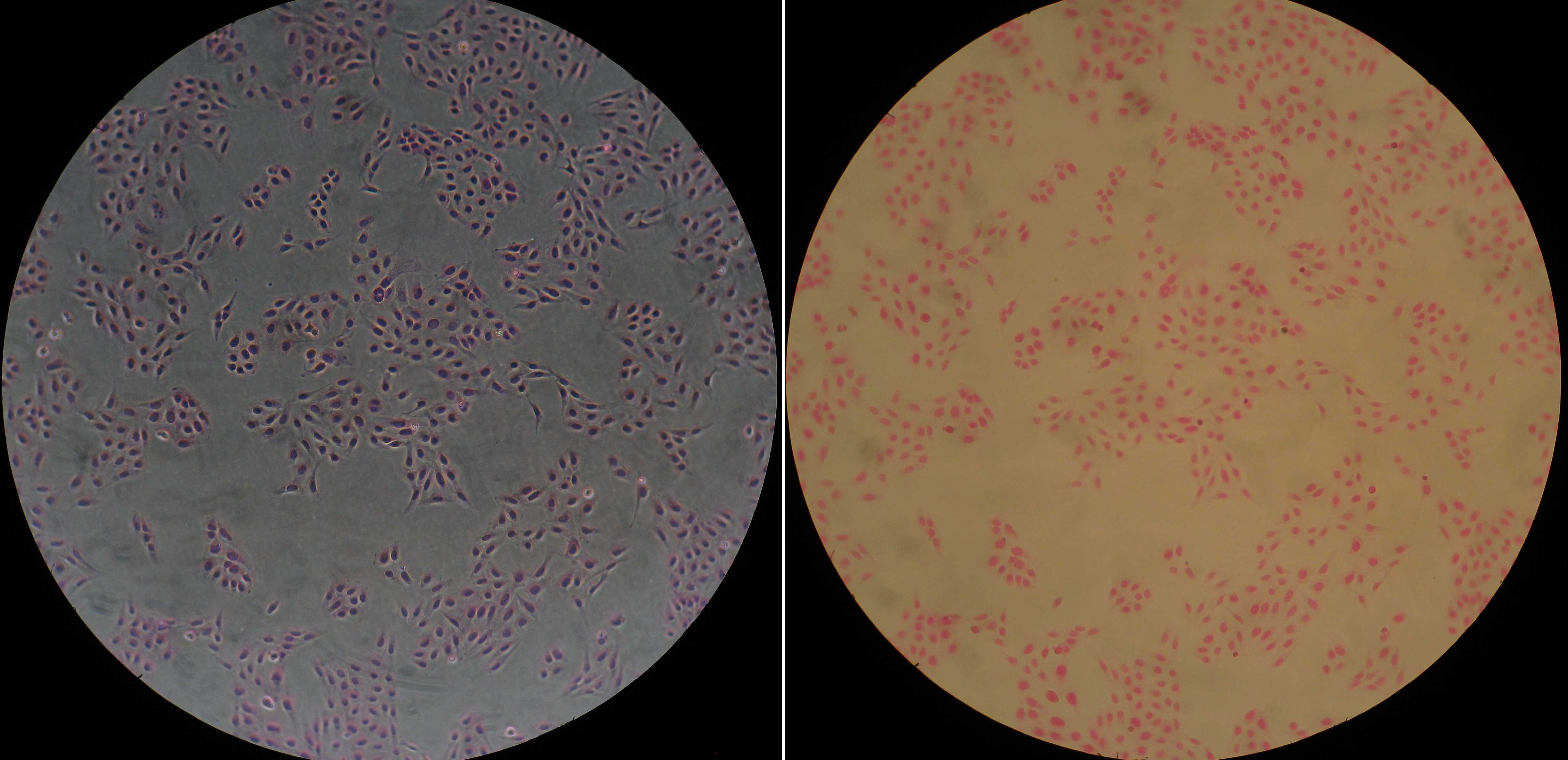
Nucleus-highlighting (terminal/lethal) staining of adherent live U2-OS cells by Erythrosine B
Misha Koksharov
Erythrosin B
vital dyes
staining mammalian cells
U2-OS
cell staining
erythrosin B
nuclear staining
protein staining
Rose Bengal
Abstract
Erythrosine B (tetraiodofluorescein; ErB) is a negatively charged viability stain commonly used to assess cell viability in a hemocytometer or in adherent mammalian cell cultures. Being considered membrane-impermeable, it preferentially stains dead cells that have lost their membrane integrity (pink color within 1 min of treatment). It is often recommended over Trypan Blue staining for providing a faster and more reliable dead cell staining and for being a generally non-toxic reagent. While exposure to Trypan Blue is toxic to mammalian cells, ErB is often stated to be relatively harmless and suitable for non-terminal cell staining. However, Erythrosine B actually enters live cells and eventually stains them lethally but the speed of this process varies considerably between cell lines and can take from several minutes (U2-OS) to more than 30 minutes (HEK293T).
In the presence of 0.06% w/v (or higher) ErB in serum-free media the adherent U2-OS cells get efficiently stained after 10 min resulting in a pronounced nuclear and a faint cytoplasmic staining while retaining the general cell morphology and adherence. This can be used for a fast terminal staining and counting of adherent U2-OS cells or other cell lines that interact with ErB in the same way.
Lower ErB concentrations in serum-free media often lead to a fast cell disintegration and loss of nuclei in U2-OS cells without staining. The discussed possible destructive and toxic effects of Erythrosine B on mammalian cells should be taken into account when using it as a vital dye. The presence of serum in the media temporarily protects live U2-OS cells from the toxic action of ErB (at concentrations below 0.1% w/v), so it can be recommended when staining dead cells to avoid affecting the live ones.
Before start
Prepare the required stock solution and media. Read the Guidelines & Warnings section describing experimental examples and the literature background of the method.
Steps
Cell staining protocol steps
Grow U2-OS cells in a desired vessel.
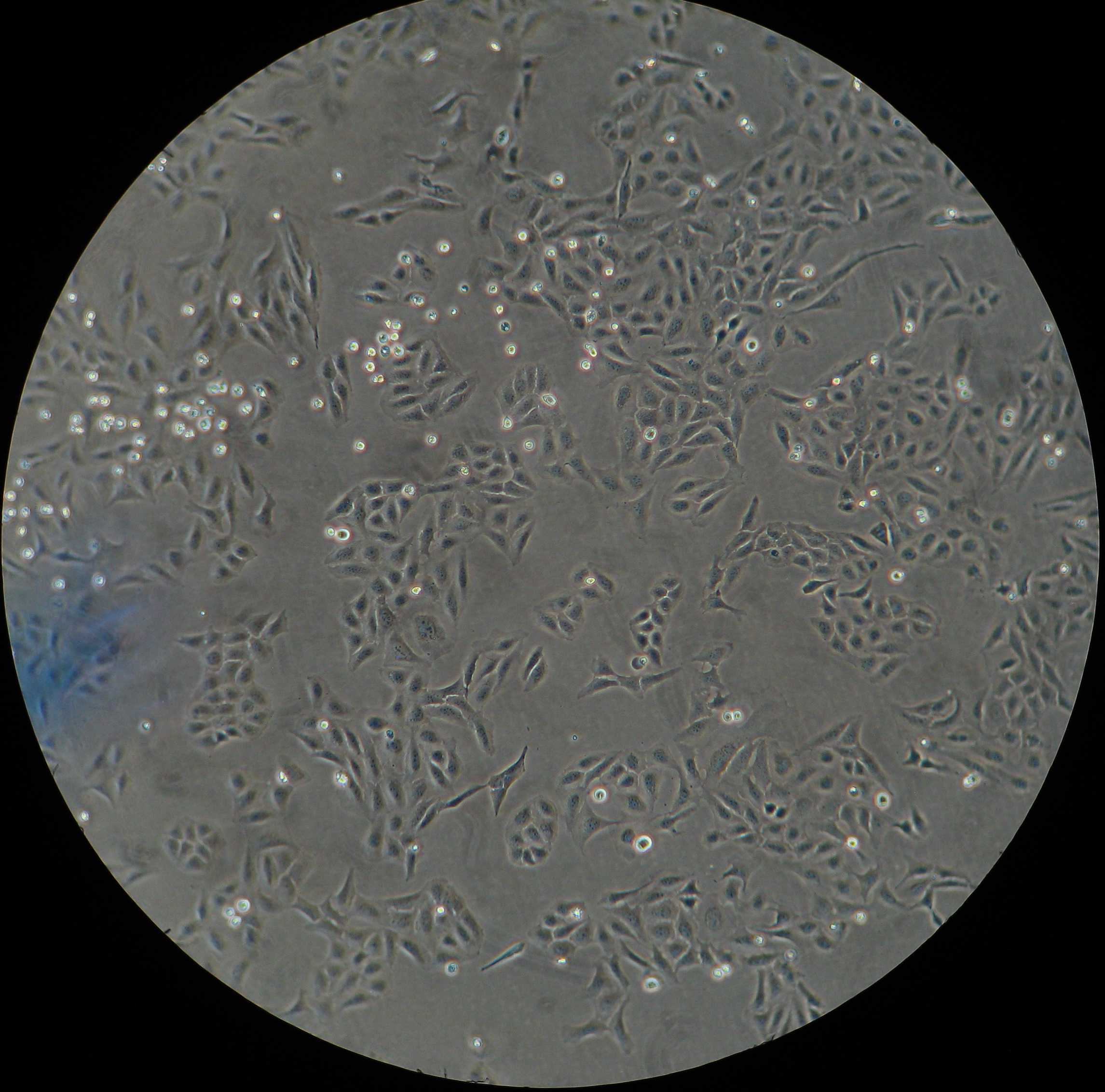
Remove the culture media from the vessel (cell culture plate or well) with adherent U2-OS cells.
Gently add a serum-free cell culture media or buffer (preferably maintaining pH 7.2-7.6 in the absence of the CO2 atmosphere) to cover the cells.
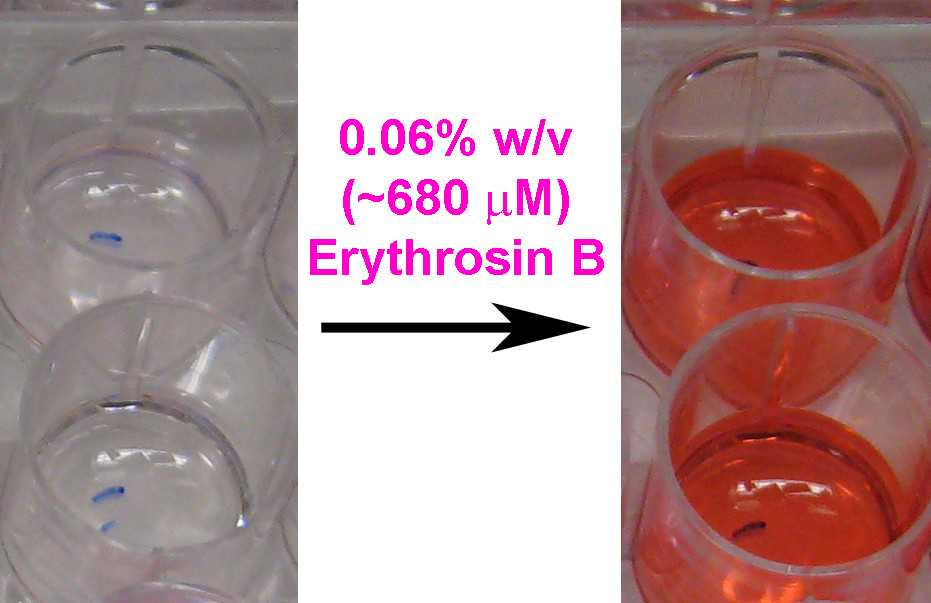
Add a small volume of 6% w/v (~6.8 mM) Erythrosin B stock (in water) to the media above cells to achieve the final 0.06% concentration (1:100 dilution). Mix by gently shaking the plate.
Incubate the plate for 10-15 min in a cell culture incubator (37°C).
The medium can be exchanged to a fresh transparent one or removed.
Cells can now be imaged in phase contrast or brightfield: nuclei become intensely pink while cytosol remains quite faint. If placed under ErB-free medium, cells will loose some of the accumulated ErB.