Non-Photochemical Quenching (NPQ) Relaxation Analysis by CF imager FluorCam FC800-331 and FluorCam 7 Software
Lynn Doran, Steven J Burgess
Abstract
For assessment of Non-Photochemical Quenching (NPQ) relaxation in leaf discs using chlorophyll fluorescence on FluorCam FC800-331 and FluorCam 7 Software under 10 minutes of low light (50 umolm-2s-1) to activate rubisco, 15 minutes of high light (1600 umolm-2s-1) to activate NPQ without causing photodamage, and 45 minutes of NPQ relaxation under low light (50 umolm-2s-1). Fluorescence measurements using saturating pulses are taken every 2.5 minutes during high light and for the first two time points after return to low light and every 5 minutes thereafter.
Before start
Prepare samples as described in "Sampling leaf tissue for analysis of NPQ Relaxation using Technologica Chlorophyll Fluorescence Imager Data. V.2".
If it is the start of an experiment set, different plant or tissue type, modifications have been made to the instrument, or modifications have been made to the protocol, prepare an additional sample plate to be dark adapted. This additional plate will be used to set the initial camera settings. Typically, once the hardware and software settings are adjusted, similar samples can be analyzed across different experiments or days using the same settings and additional plates will not need to be prepared each day. For focusing and zooming the camera, the size calibration standard can be used.
Read Photon Systems Instruments Closed Fluorcam Manual for detailed instrument setup and operation instructions. For quick start instructions, reference the PSI Quick Start Guide.
Reserve instrument using the Google Calendar. Access to the equipment reservation calendars can be requested through the IGB GEGC lab manager.
Steps
Setting up the CF Imager
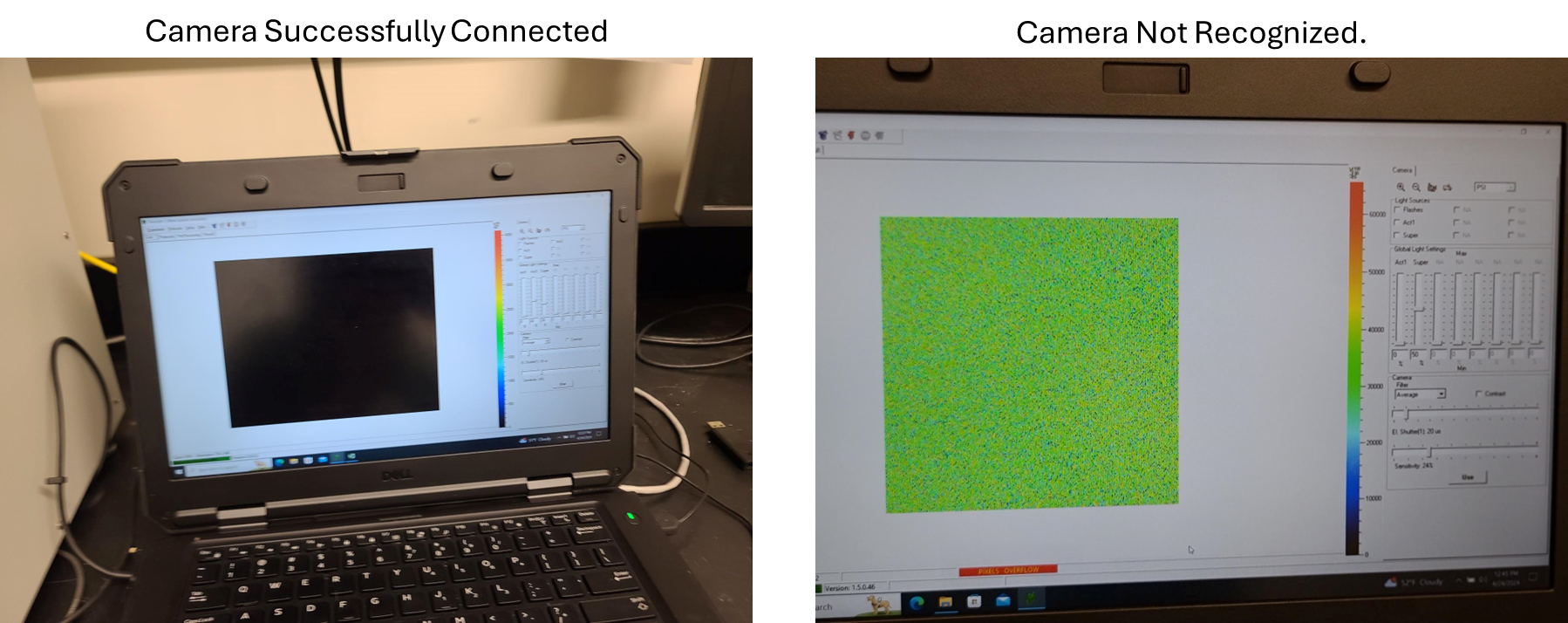
The black power supply and the white Fluorcam Instrument must both be on and fully booted for the software to recognize the Fluorcam cameras. If a black home screen appears when the software is open, the instrument is properly connected. If a yellow static screen appears, close the software, ensure both power buttons are on, wait a few minutes, and reopen the software.
If camera has already been focused, desired plate masks have been made, and the camera shutter and sensitivity settings have previously been prepared for this experiment, hardware configuration, and plant and tissue type, skip sections "Camera Focus- Performed Once at Start of Experiment", "Optional: Size Calibration (Necessary for Object Size Reporting and Using Predefined Plate Maps)", and Adjusting Camera Shutter and Sensitivity Settings - Performed Once at Start of Experiment and go straight to section "Analysis".
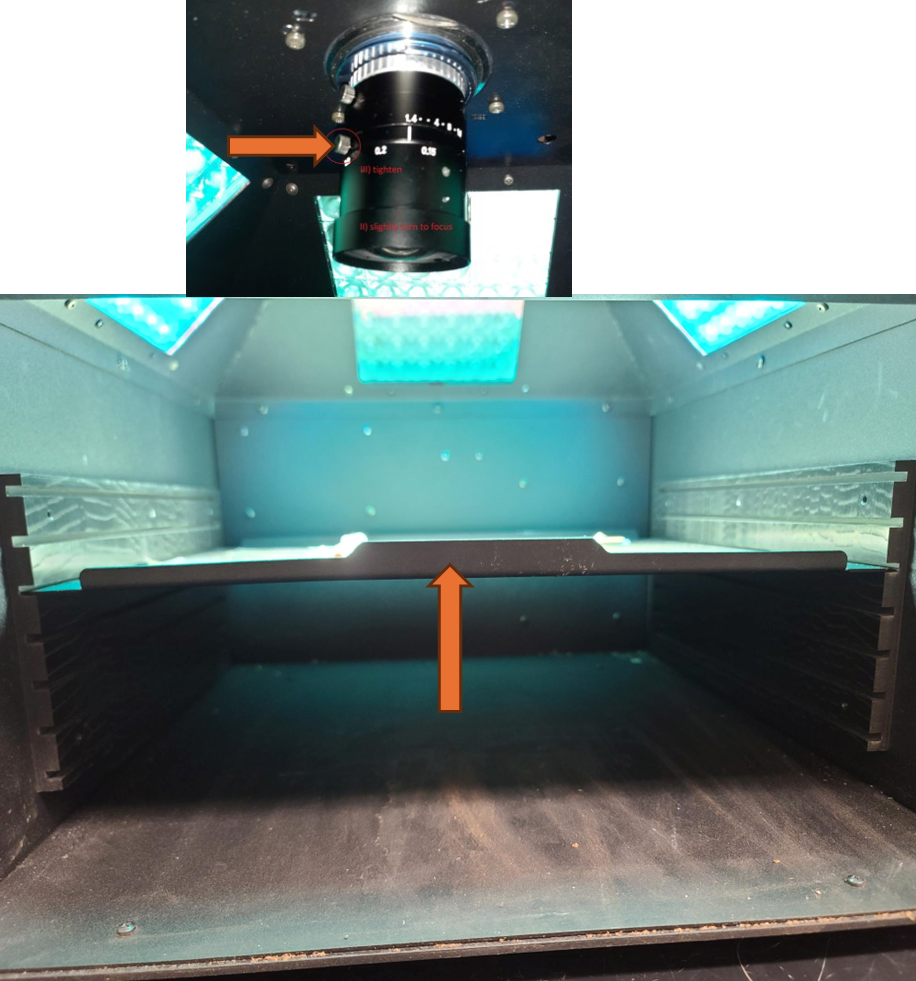
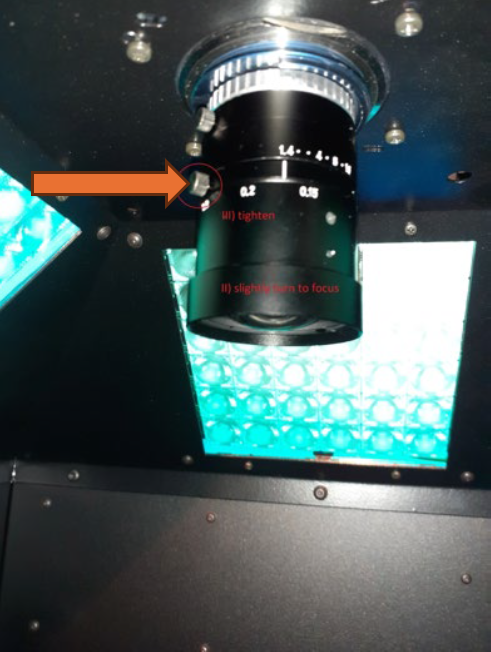
Camera Focus- Performed Once at Start of Experiment
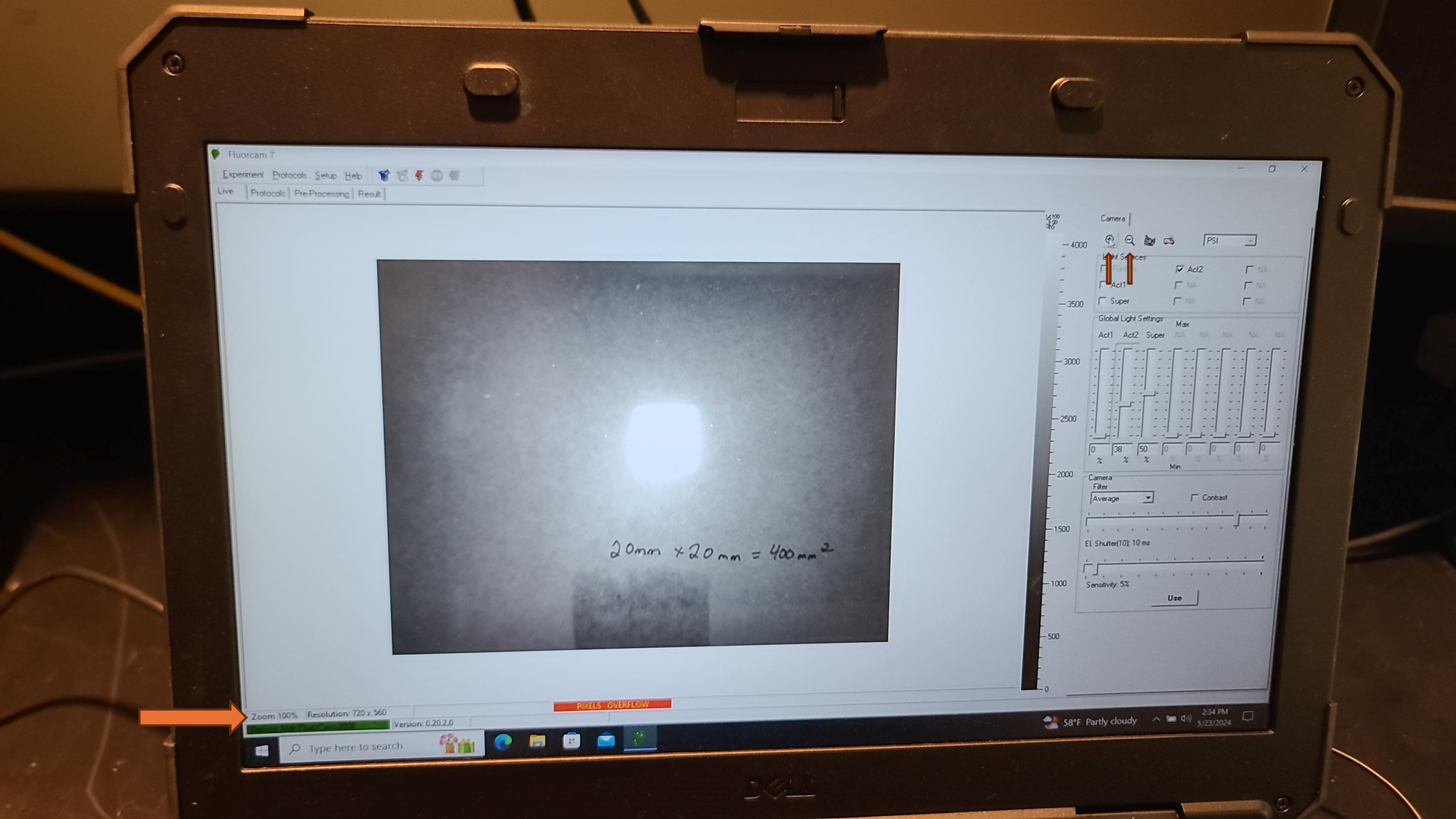
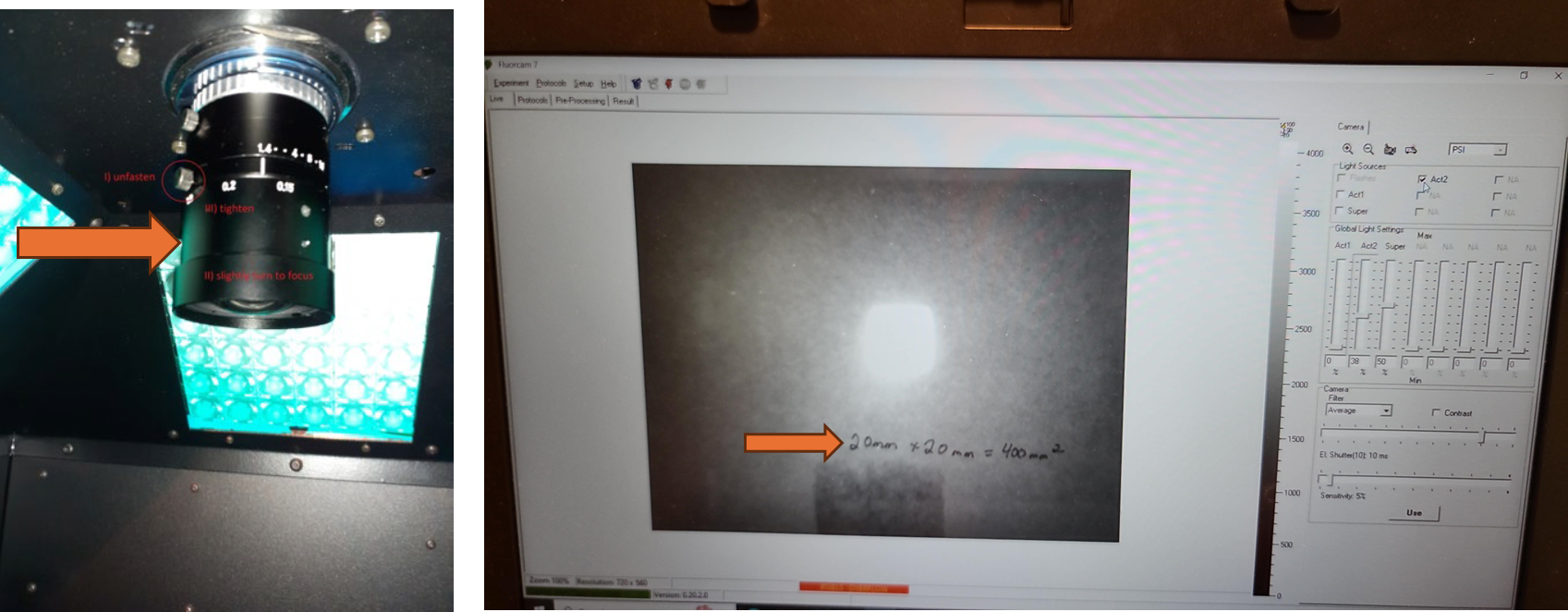
If the size calibration standard that comes with the instrument is not available, a pink fluorescent post it note can be measured and cut to exactly 20 mm by 20 mm and taped to a piece of paper. Add text to the paper to provide a focal point for focusing the camera.
Once the focus is set, do not adjust it again for the remainder of the experiment. The level of absolute fluorescence signal could change when the focus of the camera is changed.
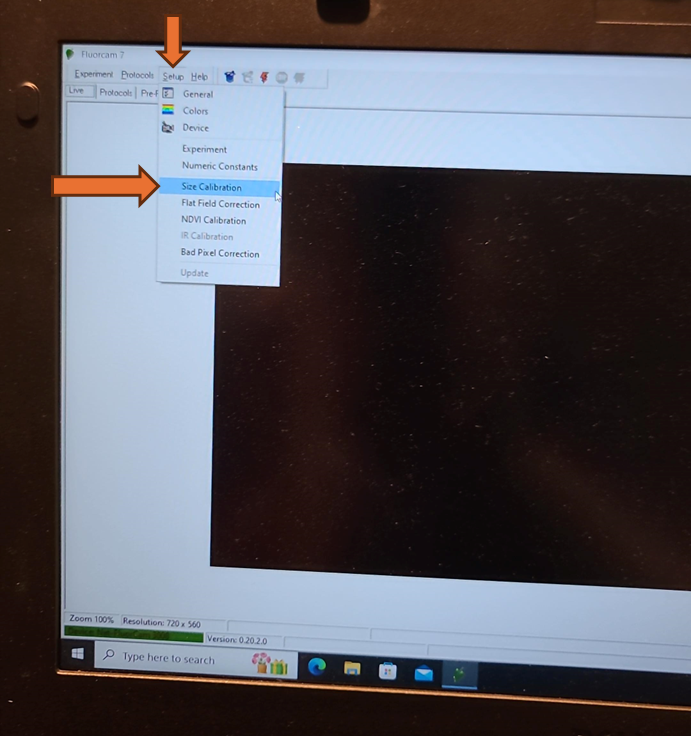
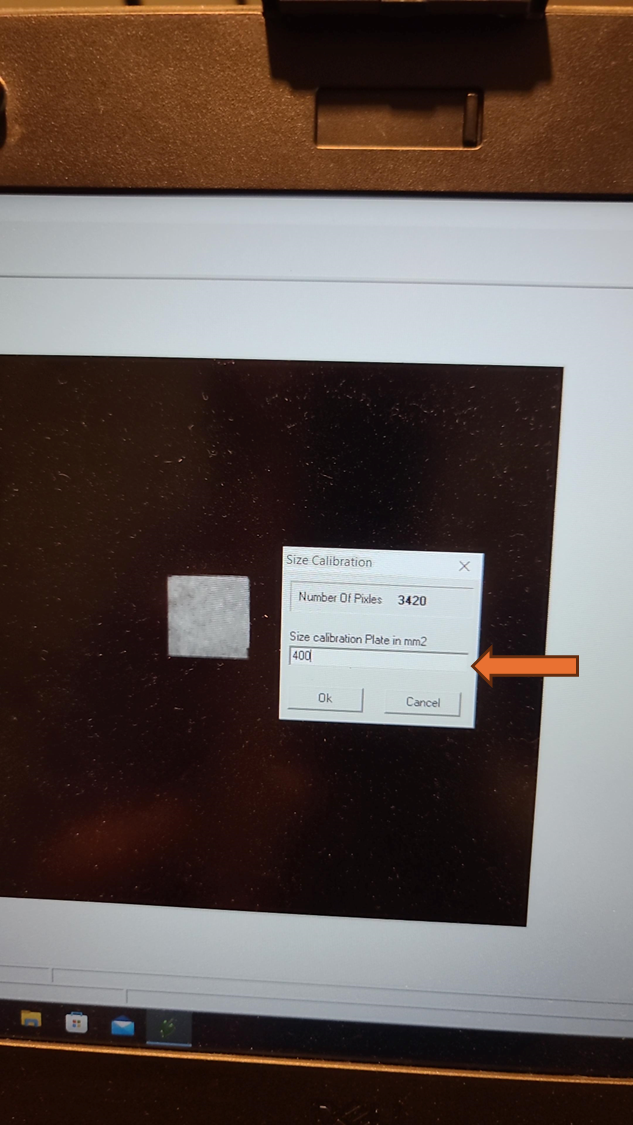
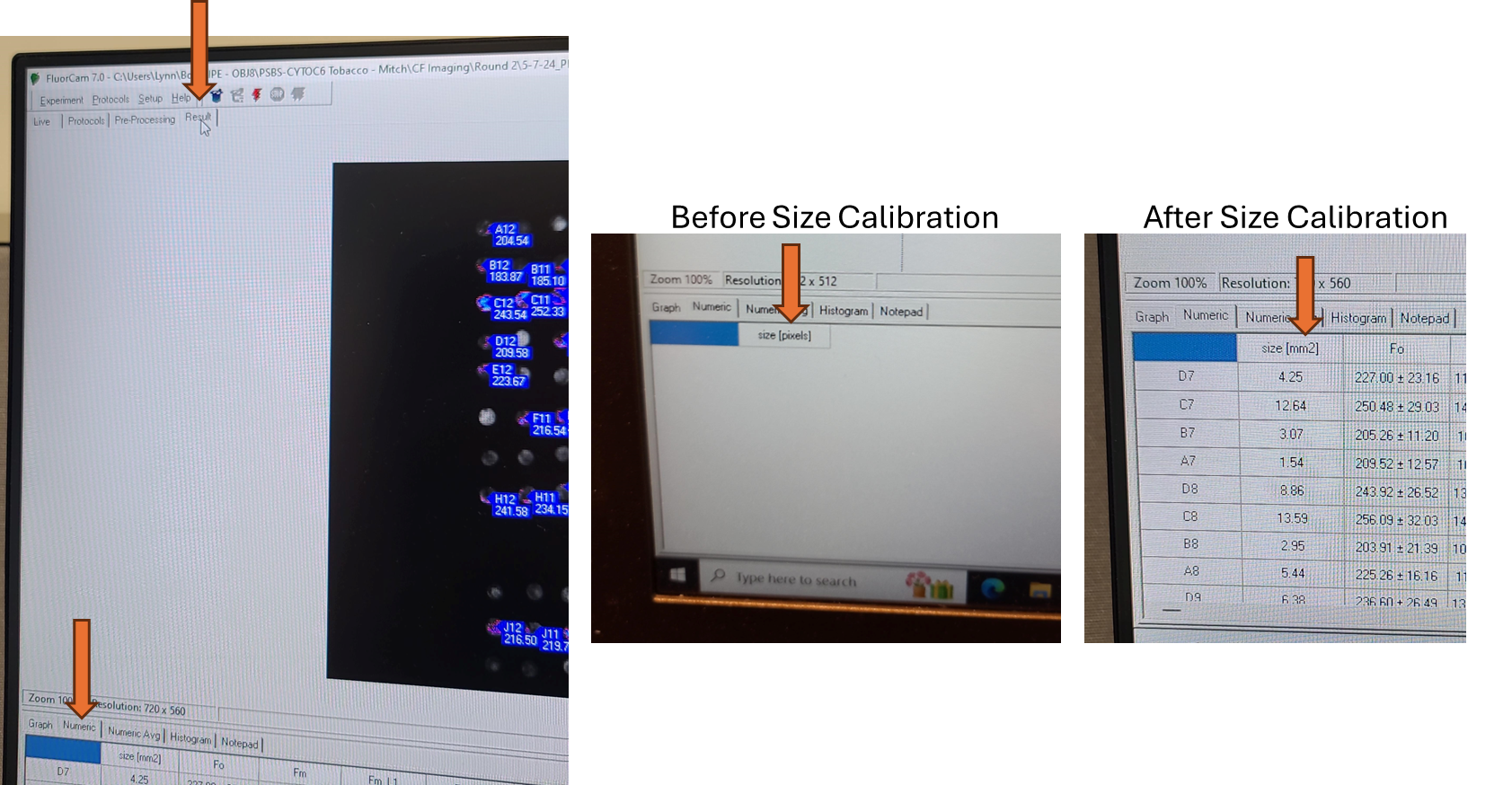
Optional: Size Calibration (Necessary for Object Size Reporting and Using Predefined Plate Maps)
If the size in millimeters of the objects measured is desired, then the size calibration must be done before each analysis. If the size calibration is being used to create a pre-processing mask (for example for a 96 well plate), it must be done before the analysis when the mask is created. Once the pre-processing mask is created, as long as the objects measured are the same size and distance from the camera, a size calibration is not needed before every analysis to use the mask.
Adjusting Camera Shutter and Sensitivity Settings - Performed Once at Start of Experiment
The shutter and sensitivity settings need to be optimized once at the start of the experiment and then should not be changed again for consistent fluorescent readings across all sample analysis runs. The shutter and sensitivity settings may need to be adjusted if plant species, tissue type, distance from camera, plant health, or plant growth conditions change affecting the baseline emission of chlorophyll fluorescence.
The goal is to adjust the shutter and sensitivity settings high enough that the camera reliably measures fluorescence at all desired timepoints under all desired conditions while avoiding pixel overload that could cause inaccurate readings and minimize the amount of additional non-treatment actinic light exposure.
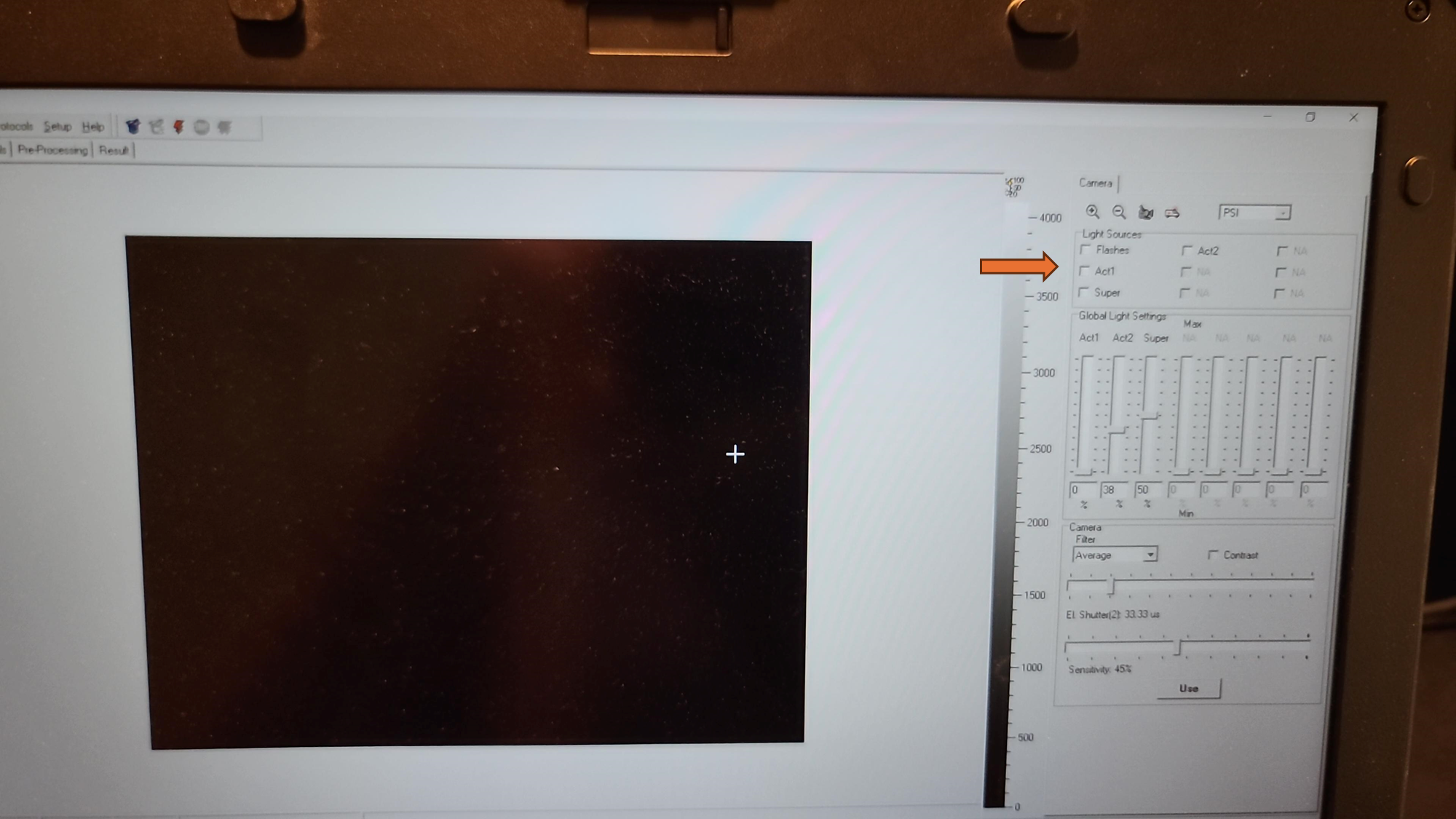
Turn off the primary overhead light and use the dimmer switch on the wall to set the lighting in the room as low as you can safely work in, with dark being ideal. Ambient light can affect shutter settings and the camera sensitivity. Leaf discs for analysis should have as limited light exposure prior to initial fluorescence measurement as possible.

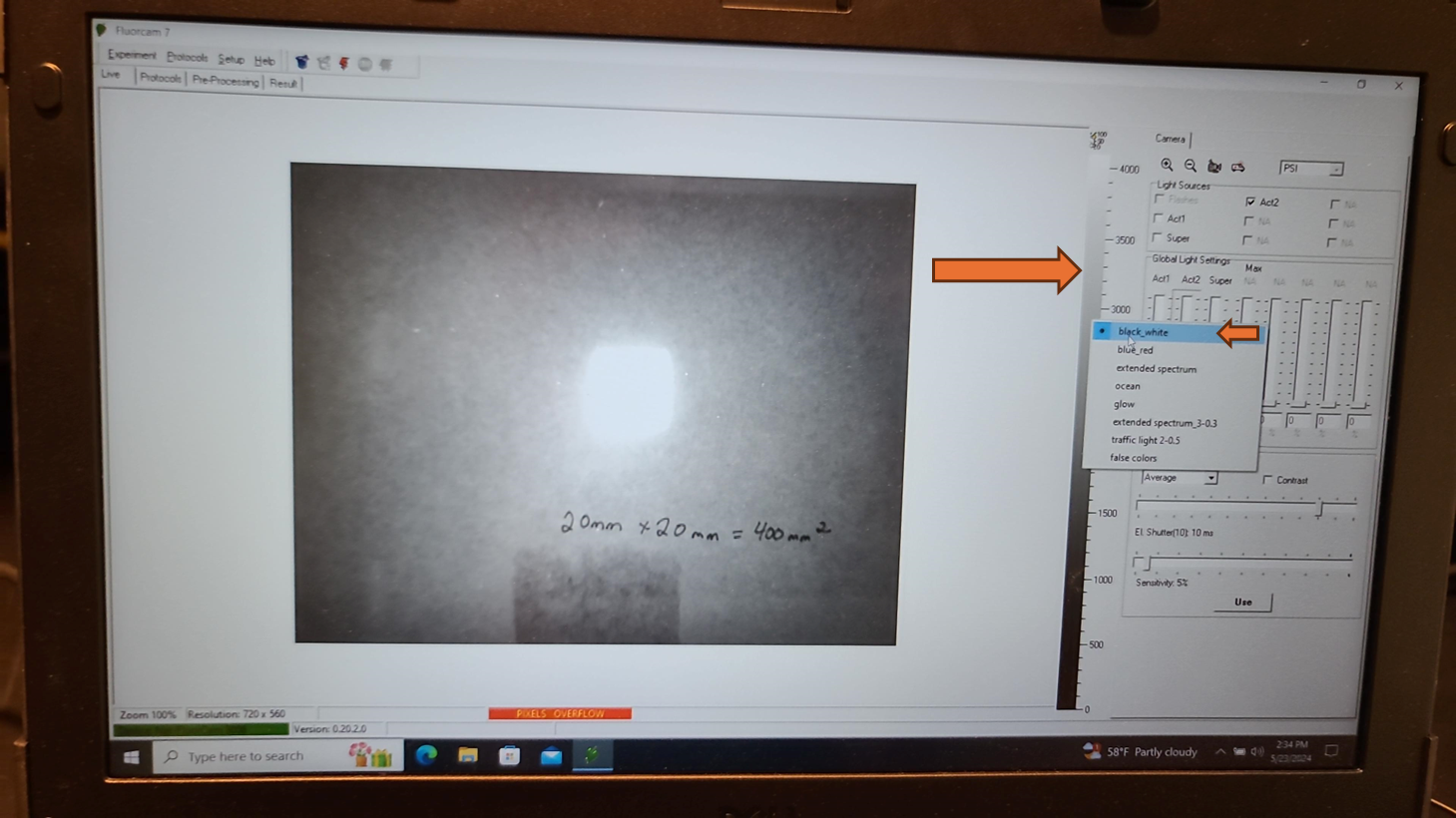
Remove any aluminum foil and parafilm from the additional sample plate that was prepared and dark adapted (from "Before Start" section), flip the plate upside down so the adaxial side of the leaf faces towards the Fluorcam camera, and place on the shelf with the corner of the plate snugly against the guardrails for the field of view.
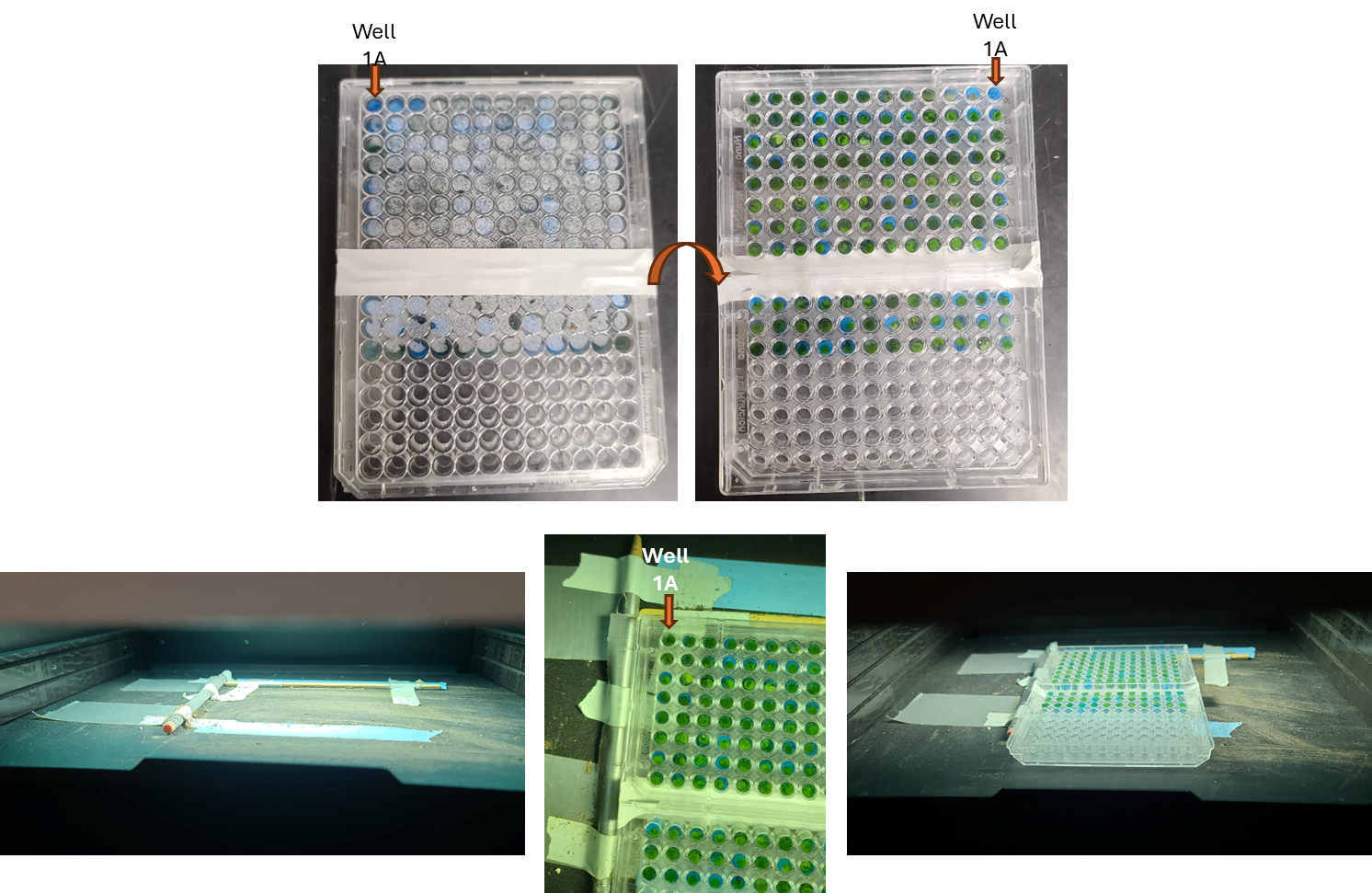
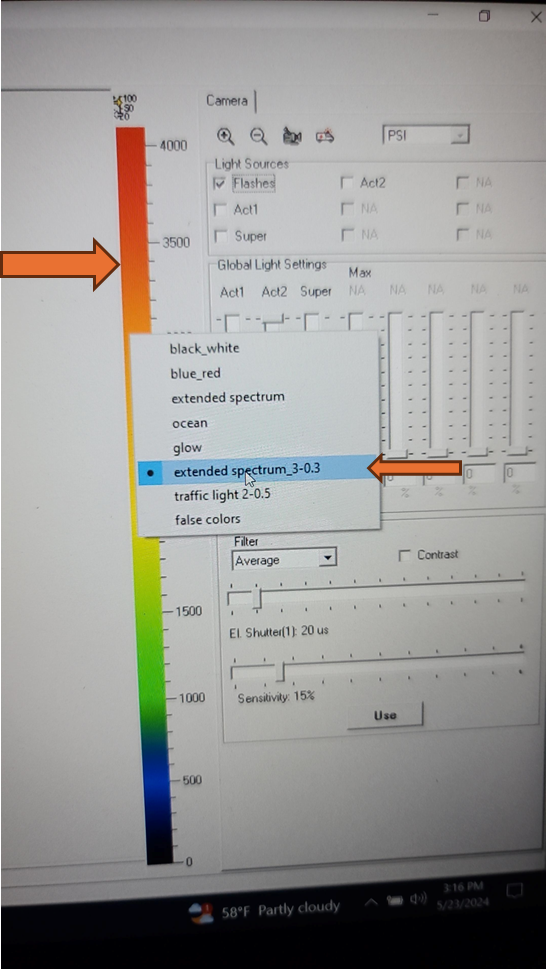
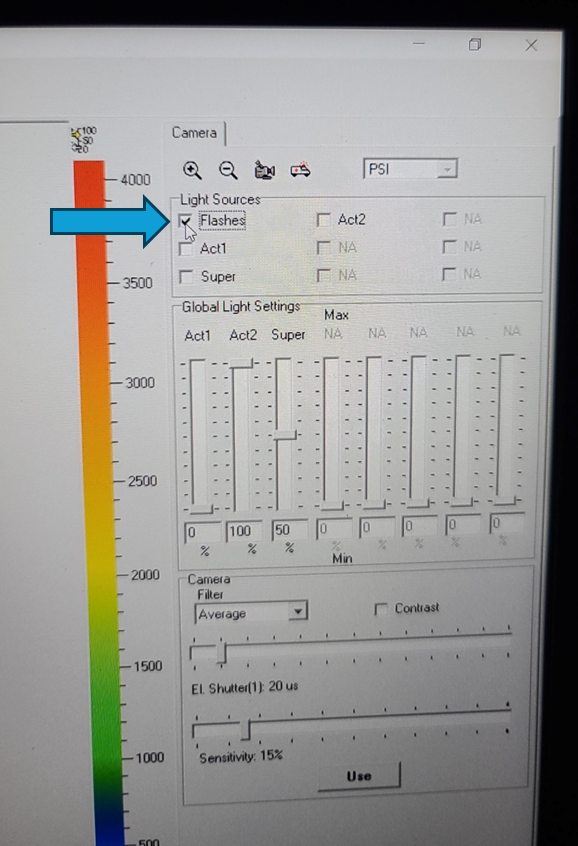
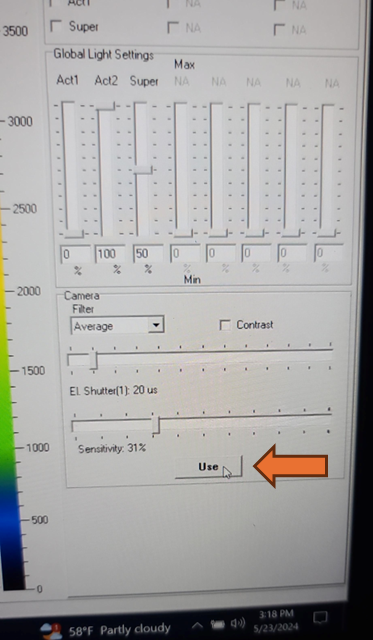
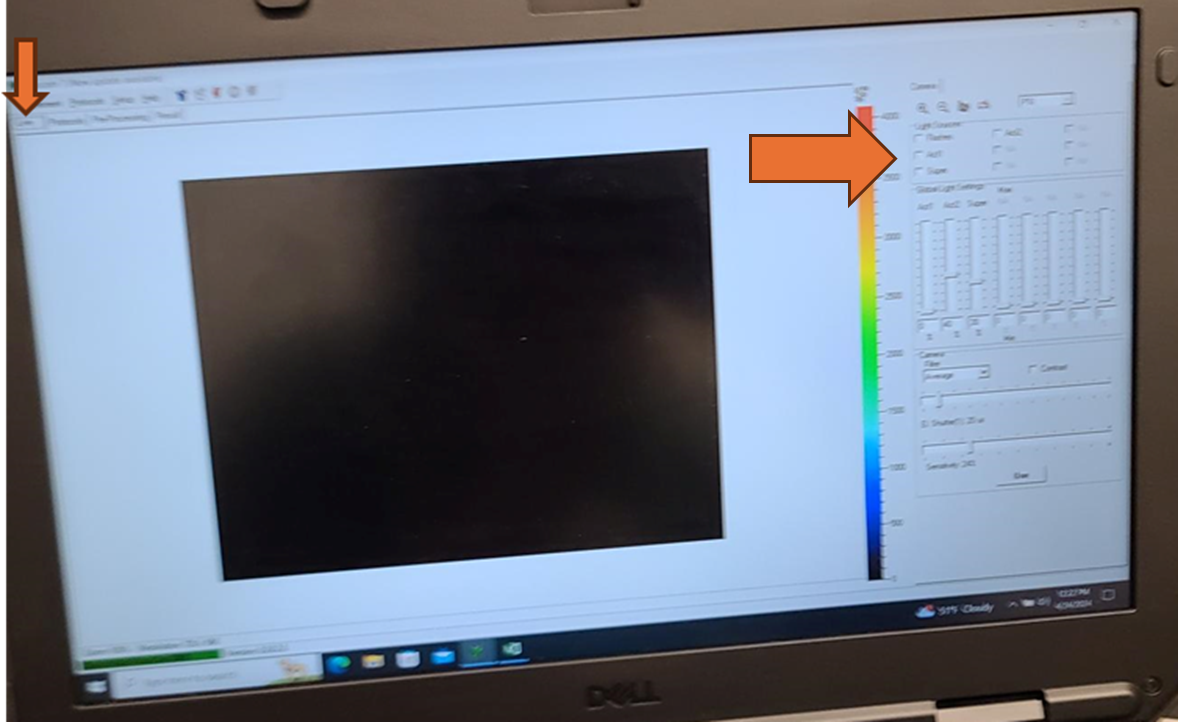
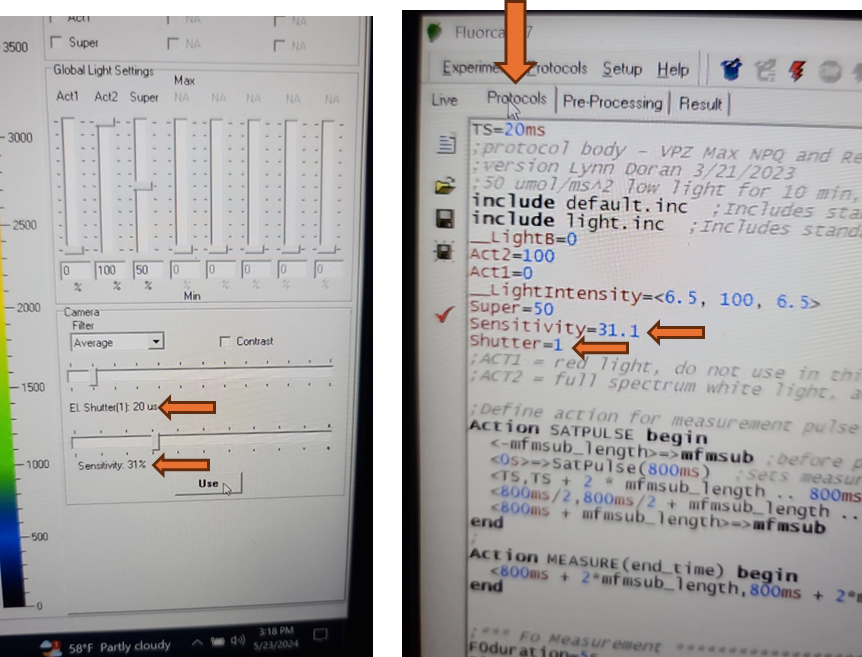
Set the EI shutter slider bar to 1 and adjust sensitivity slider bar until the leaf discs (or other plant tissue) is visible in the extended color scale and are a blue corresponding to between 200 and 500 on the color scale to the right of the live window. If it is not possible to get the tissue to appear on the highest sensitivity and EI shutter 1, increase EI shutter to 2 and repeat. The goal is to keep the EI shutter as low as possible to reduce unintended actinic light treatment.
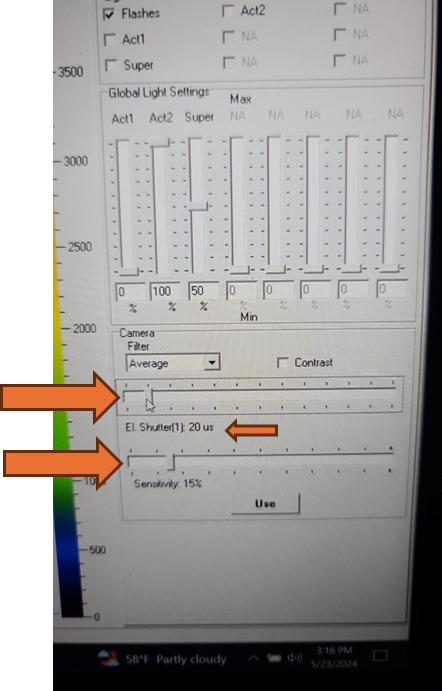
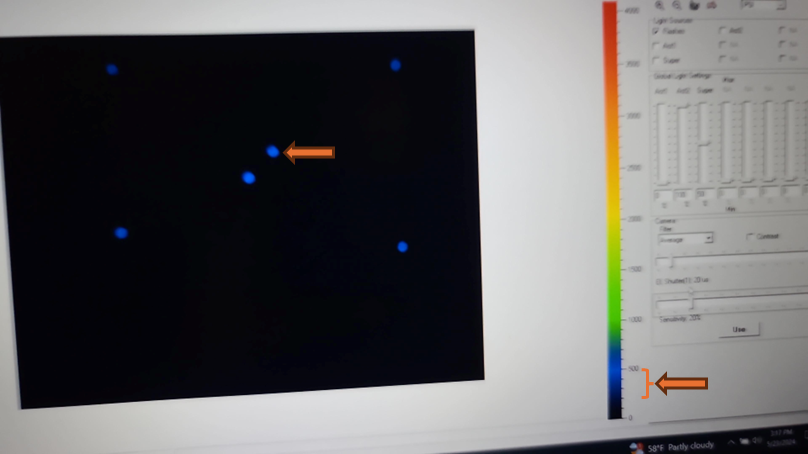
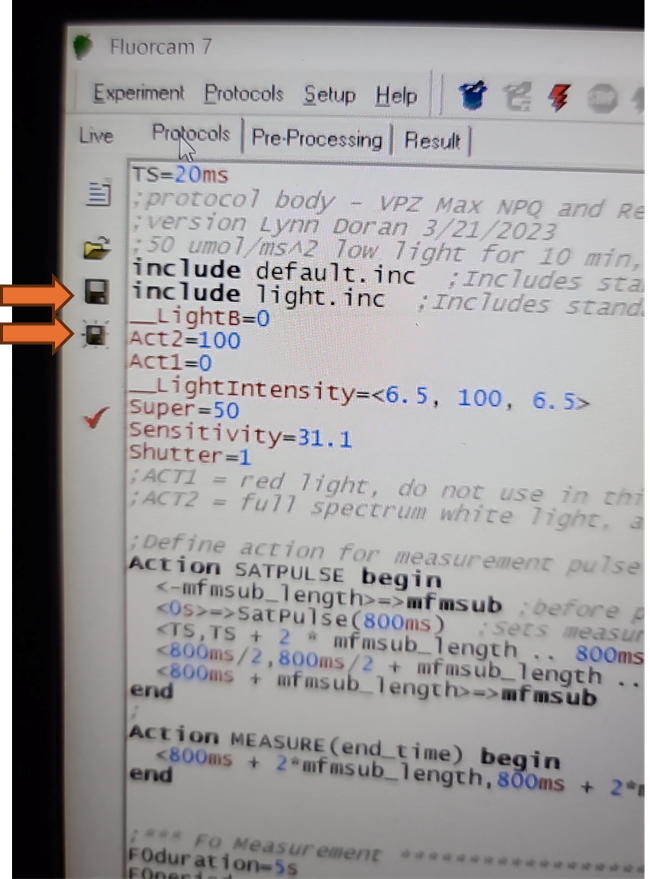
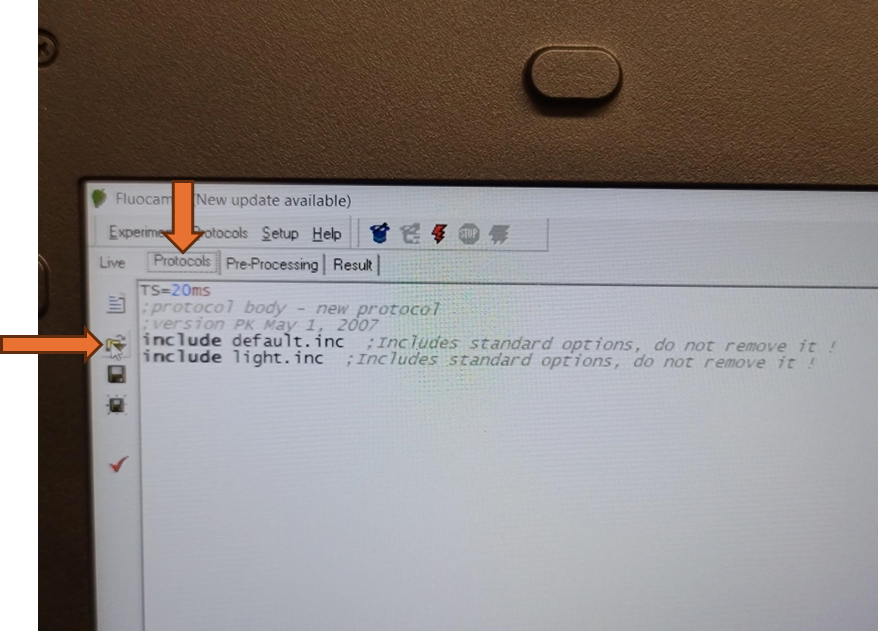
The updated EI shutter and sensitivity settings need to be imported into an existing protocol. Navigate to the protocols tab above the live field of view and use the open folder icon to select the desired protocol from the computer file system.
Protocol for NPQ relaxation:
VPZ-NPQ_50umol-10m_1600umol-15min_50umol-45min_annotated_v2.p
Alternatively, a pre-defined Fluorcam protocol can be selected and configured from the "Protocols" tab by selecting the wizard hat icon.
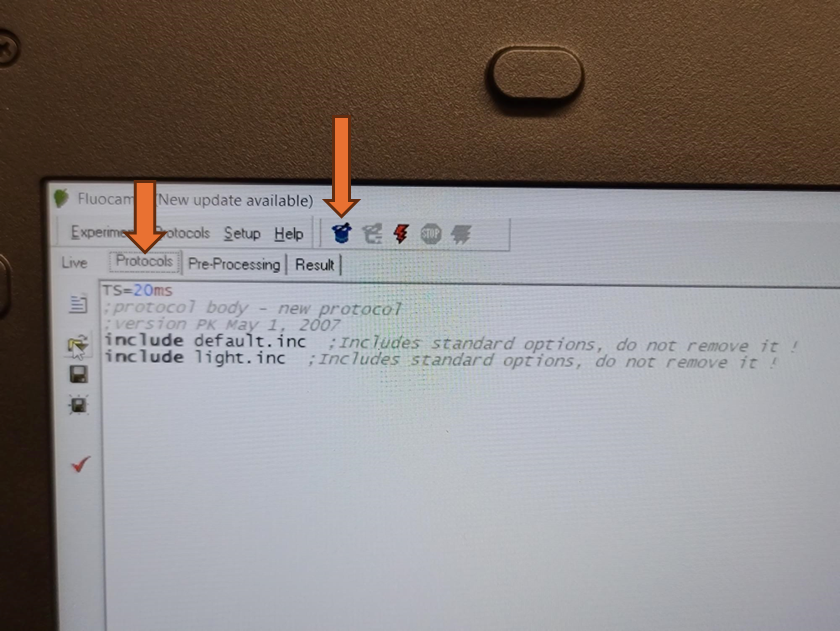
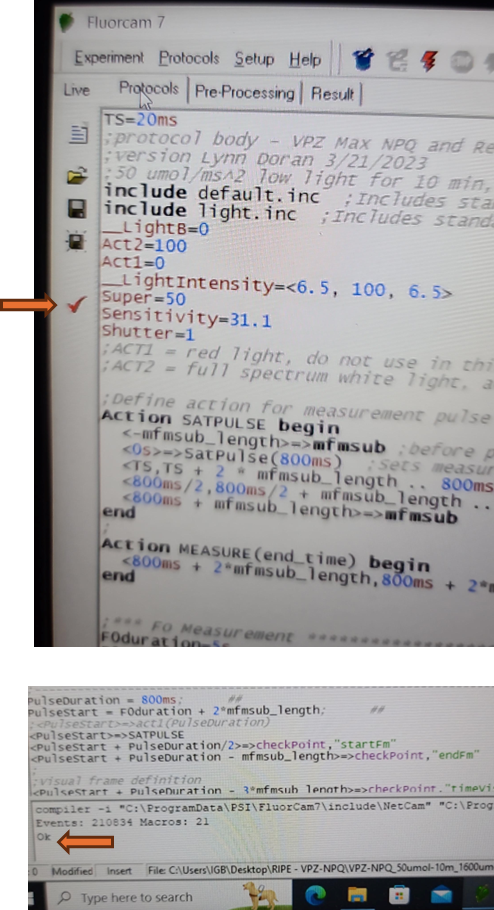
Once a protocol is successfully loaded, the coding section of the screen should populate. Be aware that the save and save as icons on the left side of this screen will only save modifications made to the loaded protocol. It will not save any of the size calibrations or camera settings in the live window.
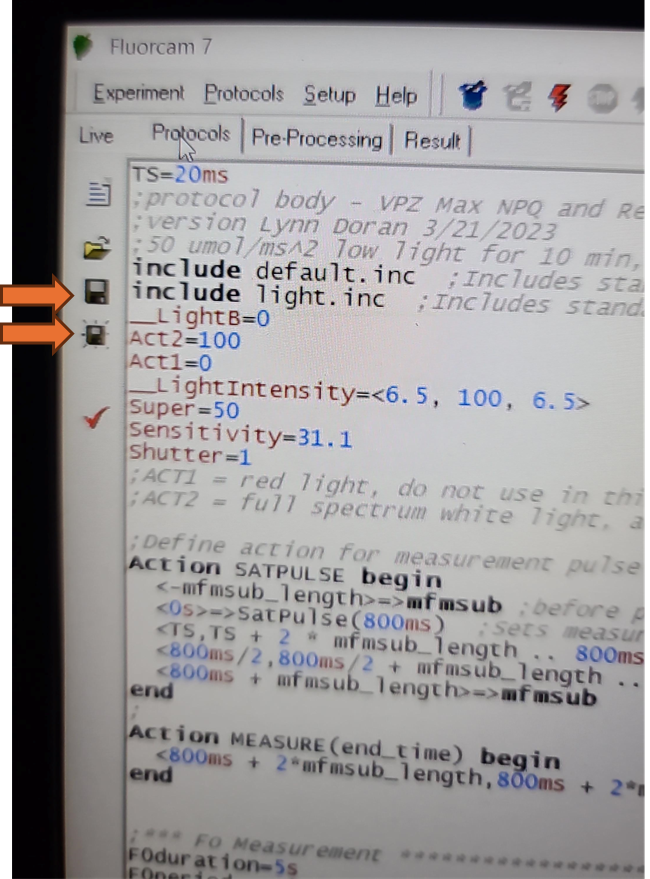
Verify that the EI shutter and sensitivity settings were successfully imported into your protocol. Take note of the EI shutter and sensitivity numeric values on the Live window. Navigate to the protocols page using the "Protocol" tab at the top of the live field of view. Verify that the protocol code at the top of the method for Sensitivity and Shutter match the values that were selected on the Live window.
Analysis
If applicable, remove the sample plate that was used for adjusting camera settings. It is no longer dark adapted and should not be used for real measurements.
Turn off the primary overhead light and using the dimmer switch on the wall, set the lighting in the room as low as you can safely work in, with dark being ideal. Ambient light can affect shutter settings and the camera sensitivity. Leaf discs for analysis should have as limited light exposure prior to initial fluorescence measurement as possible.

If used for dark adaptation, remove aluminum foil from sample plate.
If used, remove parafilm from plate edges. Humidity should be maintained for analysis by the sponges and any parafilm covering a leaf disc could interfere with light treatments and saturating pulses.
The Fluorcam is not configured with an auto-save. Save the complete experiment .tar file by selecting Experiment-> Save from the top menu bar.
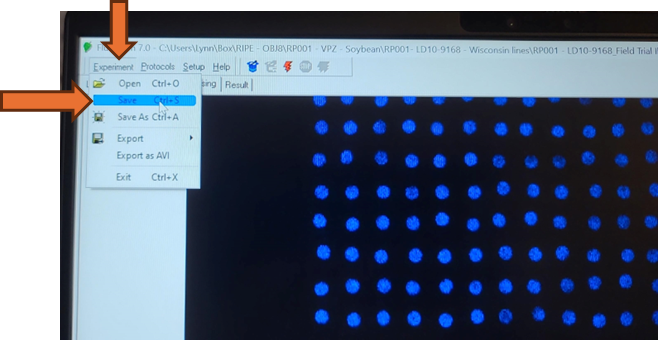
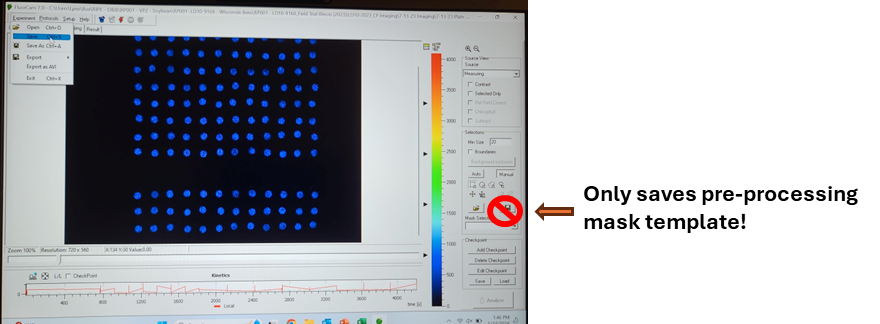
Do not use the save icon on the Pre-Processing window. The save icon on the Pre-Processing window will only save a pre-processing mask and all run data will be lost.
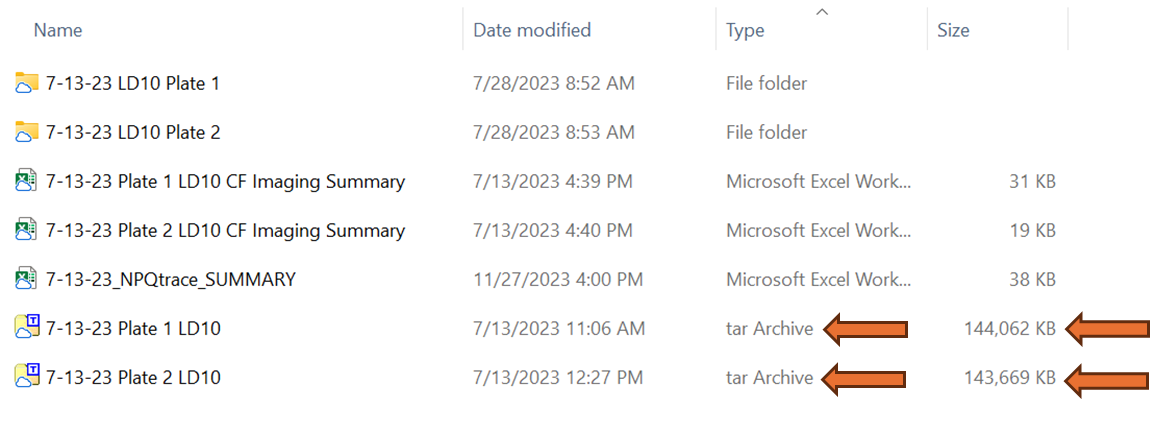
Verify that the file was saved correctly by navigating to the PC location where the analysis file was saved. The file should appear as file type "tar Archive" and be approximately 140,000 KB file size if the provided NPQ protocol was used. This tar file contains the size calibration, protocols, and all analysis imaging data. If a mask was applied or result analysis was initiated it will also contain that data but these two actions can also be performed at a later time.
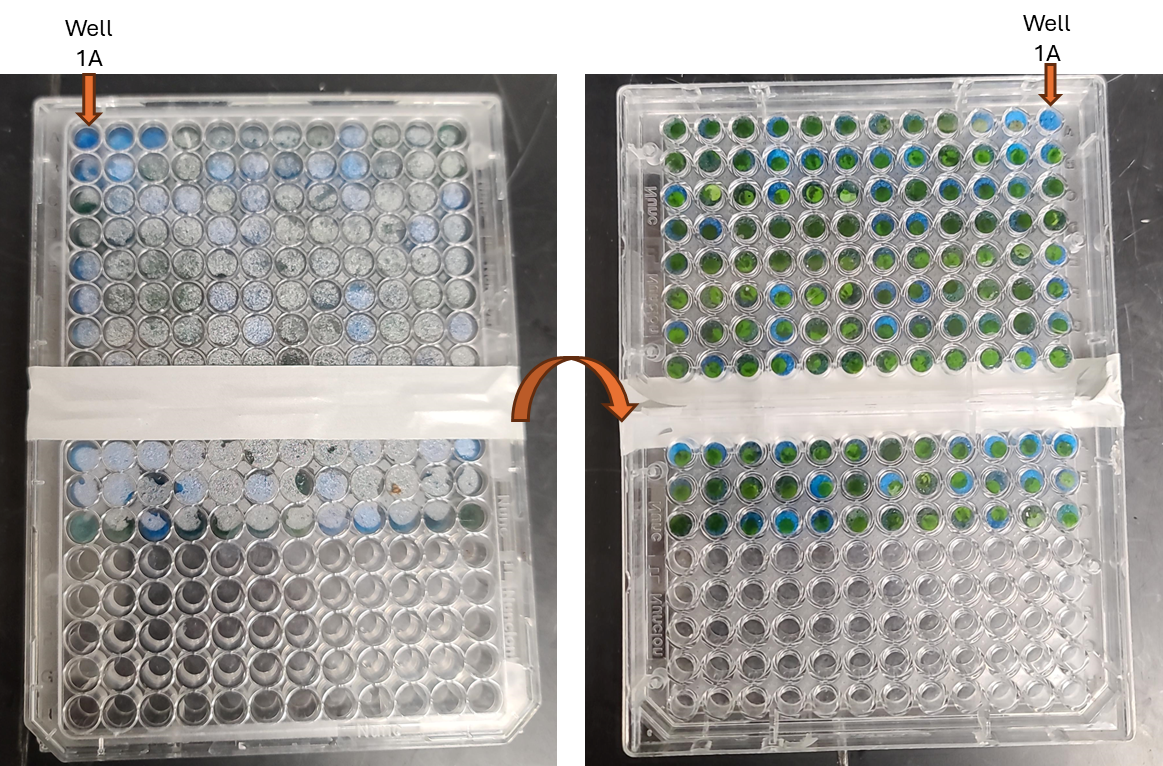
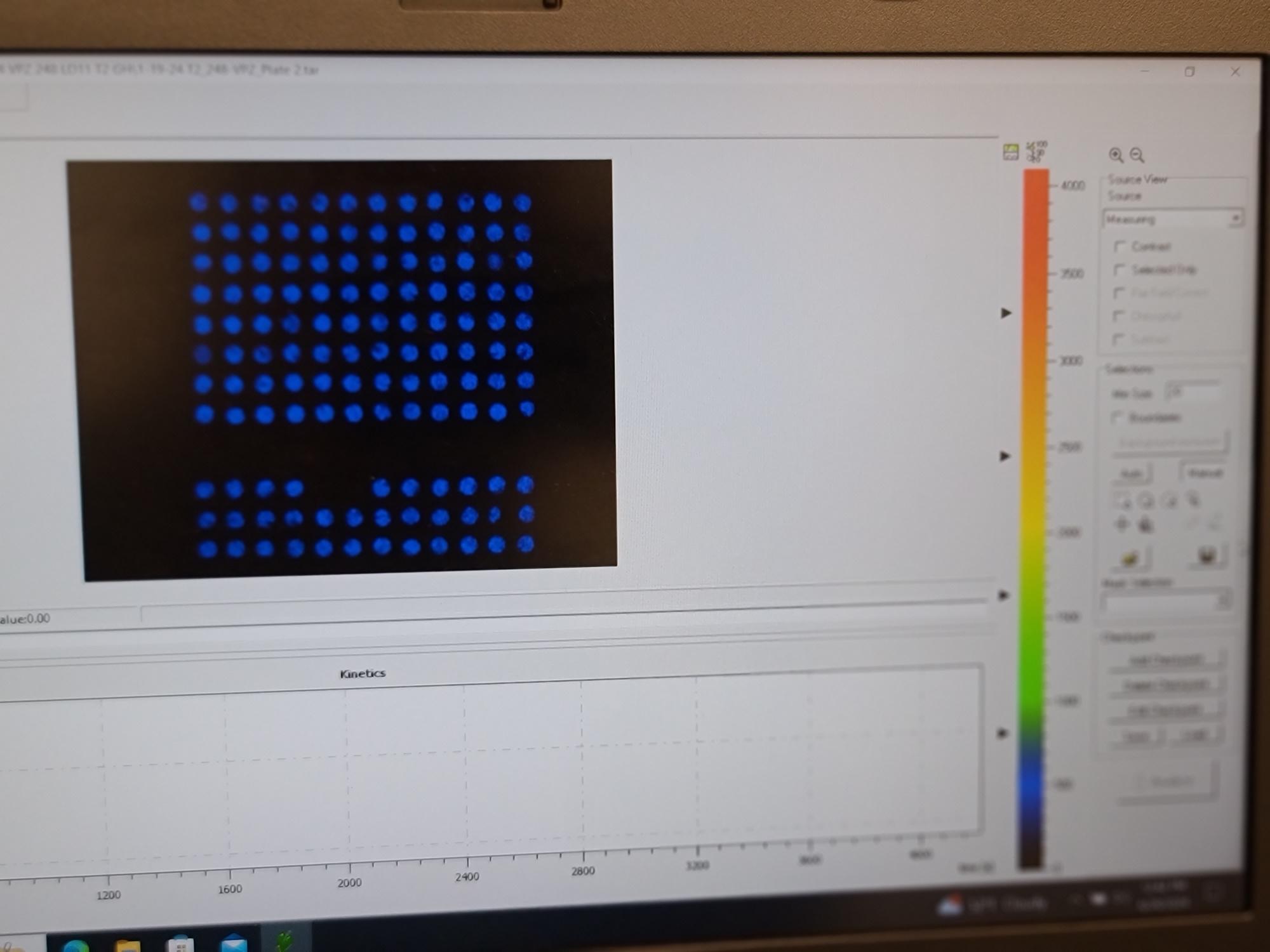
Remove the completed sample plate from the imager and confirm that well 1A was in the top right corner and well 12A was in the top left corner and that the file name matches the plate analyzed.
Analyze the next plate or sample set following the same steps. The software does not need to be cleared. Once the next plate is ready for analysis, select the lightening bolt icon to begin. Be aware that as soon as the new analysis has begun, the previous pre-processing and results will not be accessible from the file being live generated. When this analysis is complete, be sure to use "save as", not save, to avoid overwriting the previous analysis. For added security to avoid overwriting analysis runs, the software can be closed completely but the size calibration and loading of the protocol will have to be repeated every time the software is closed and then opened.