MIBI: MIBI staining of fresh-frozen/OCT-embedded samples
Sven Truxa, Felix J Hartmann
Abstract
This protocol entails the recommended staining procedure for Multiplex Ion Beam Imaging Time of Flight instrument (MIBI_TOF) as developed in the Sean C. Bendall and Michael R. Angelo labs, and has been adapted in the lab of Felix Hartmann specifically for the staining of fresh-frozen samples.
Before start
Verify the stocks of all reagents and place an order or prepare solutions, if some reagents are running low.
Steps
Initial comments
If using frozen and vacuumed slides (e.g. from collaborators), let slides come to room temperature before destroying the vacuum seal ! (prevent condensation!) ~5-10min
In the morning: heat up PT Module and prepare fresh antigen retrieval solution (step 6) to place in the device for preheating
Buffer preparation
Prepare 1x PBS by diluting 20xPBS 1:20 in Ultrapure type 1 water (needed for blocking buffer)
Prepare Blocking buffer (also used as antibody diluent in this protocol!)
This buffer does NOT contain Tween!
| A | B |
|---|---|
| Reagents | Qty for 5mL |
| PBS 1x | 4750µL |
| Horse Serum | 250µL |
Constituents of blocking buffer
Filter with .45µm syringe
Verify stock of 1x PBS wash buffer and prepare accordingly if running low
This buffer does NOT contain Tween!
| A | B |
|---|---|
| Reagents | Quantity of 250mL |
| PBS 10x (ml) | 25mL |
| Bovine Albumin (BSA), heat shock treated (g) | 0.25g |
| Ultrapure (type 1) water (mL) | 225mL |
Constituents of PBS washing buffer
To prepare fresh antigen retrieval solution , dilute 10x DAKO (3-in-1) pH9 antigen retrieval solution 1:10 in ultrapure (type 1) water (e.g. for 25mL: 2.5mL + 22.5mL)
note: if retrieving in gold slide delivery chamber (5 slots), 25mL are sufficient
| A | B | C |
|---|---|---|
| Total volume (mL) | Volume target retrieval (mL) | Volume (mL) ddH2O |
| 25 | 2.5 | 22.5 |
| 50 | 5 | 45 |
| 100 | 10 | 90 |
Dilution table for Dako Antigen retrieval solution
Thaw and fix, wash
If embedding medium is still on slide, carefully dip slide into 1x PBS before fixation
Fix Slides after thawing in 10% Neutral Buffered Formaline (NBF) for 1h at room temperature
Transfer the slides in the first MIBI 1x PBS wash buffer and dip shortly to remove PFA
Transfer the slides to the second 1x PBS wash buffer and dip shortly to remove PFA
Antigen retrieval after fixation
After washing, transfer slide into antigen retrieval buffer jar within the preheated PT module

press run on the digital screen as soon as the device is preheated to 75°C. The display will show "WARMUP" and heat up to 80°C for retrieval , stay at this temperature for 20minutes, direcly put tissue back to RT.This process takes ~30min.
Take slides out of the PT module and let them cool to room temperature (~10 min) before proceeding.
Sequenza assembly
Fill a disposable Pipetting Reservoir with 20mL of 1x PBS wash buffer
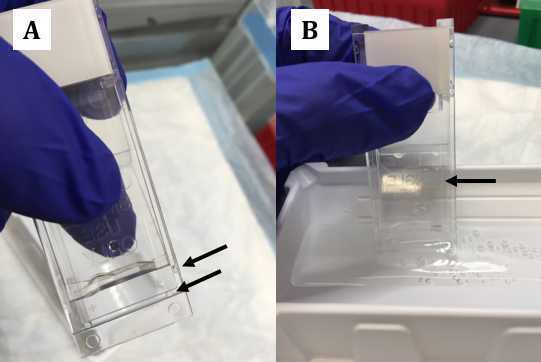
Fill by capillarity the space between the slide and cover plate by holding the parts tight and dipping the bottom part of the assembly in the wash buffer reservoir (see picture above panel B).
If capillary force is not sufficient to fill the staining reservoir, a careful pumping motion on the triangular protrusion can help.
Secure the assembly and make sure that the assembly placed down in the rack (see above picture panel D)
Add 1mL of wash buffer. The buffer should flow thru within 1 min 30 s. Repeat by adding 1 mL of wash buffer
Blocking
Add 200µL of blocking buffer to the sequenza assembly, make sure the buffer is retained in the assembly (the waterline should stop at the upper end of the capillary space)
Incubate at Room temperature for 1h 0m 0s
Multiplex Antibody mix
Prepare antibody mix based on the putative multiplex antibody panel
Make sure that all the antibodies are ready to use BEFORE starting to build the panel
It is highly recommended to prepare all the antibodies, ready to use, a day before the panel is built
The total volume needed for staining each Sequenza slide assembly is 120µL
Build an antibody mix table information to make the antibody panel as follow:
Conjugation ID, Target name, Channel, Antibody concentration, Titer, Volume
Exemple:
The total volume needed for staining each Sequenza slide assembly is 120µL
| A | B | C | D | E | F |
|---|---|---|---|---|---|
| ID | Target | Channel | Concentration µg/mL | Titer (µg/mL) | Volume (µL) |
| 1565 | CD45 | 169 | 50 | 0.25 | = |
| 1516 | CD8 | 158 | 50 | 0.5 | = |
| ... | ... | ... | ... | ... | ... |
| Total | 150 | ||||
| Antibody mix | =F5-F7 | ||||
| Blocking buffer | =F5-SUM(F2,F3) |
For Blocking Buffer solution preparation refer to step 4
Prepare the antibody mix according to the calculation. Make sure to spin down antibody vials before pipetting to prevent pipetting of aggregates. Use filter tips when pipetting from communal stocks and keep the tubes on ice.
Pre-wet spin column
Add 400µL of clean blocking buffer to a Centrifugal 0.1 µm filter unit (Millipore, UFC30VV00)
10000rcf , discard flowthrough
Add antibody mix to the filter unit
10000rcf
Stain 1 (Overnight)
Add filtered antibody master mix (120µL) onto the sequenza assembly
Place the moist chamber at 4˚C , preferably in a place with low disturbance
Post staining washing step
Following overnight incubation, wash twice with 1 mL of wash buffer
After the 1h incubation, wash twice with PBS wash buffer within the sequenza assembly by pipetting 2x 1mL.
Prepare solutions
Prepare fresh 2% glutaraldehyde fixing solution
Glutaraldehyde fixing solution
- Add
30mLof 1x PBS low barium in a 50 mL tube - Break the glass glutaraldehyde 8% (amber vial)
- Add the glutaraldehyde (10 mL) to the diluent by inverting it and tapping the bottom of the vial
- Transfer the content in a linear stainer container
Set the linear stainer containers
Fill containers with the following solution and order
Glutaraldehyde x 1, PBS low barium x 1, TRIS 100 mm pH 8.5 x 3, ddH2O x 2, 70% Ethanol x1, 80% Ethanol x1, 95% Ethanol x 2, 100% Ethanol x 2, exit stainless steel tank = empty
Glutaraldehyde fixation
Disassemble the sequenza setup. Make sure at this point that the slide doesn't dry out and directly proceed to the next steps.
Mount the slides on the linear slide holder
Fix in 2% glutaraldehyde for 0h 5m 0s
Rinse briefly with 1x PBS low barium
Dehydration and Storage
Press on Menu
Check for Processing time = 30 sec, Lift bar = 976, Number of dips = 3
Continue to press Menu until the screen displays Start at: __
Set Start position corresponding to the first slide carrier position
Exemple: If the first slide carrier is at position 3, use Plus ( + ) or Minus ( - ) button to increase or decrease to get Start at: 03
Then press Enter
Press Run on the Linear Stainer
Allow the dehydration process and wait until the slides reached the empty stainless steel tank and stop
Store the slides immediately under vacuum until MIBI acquisition
Alternatively, the stained slides can be stored in a vacuum sealed bag for longterm storage pre and post MIBI acquisition