Lysosomal GCase (glucocerebrosidase) activity assay
Sara Gomes, Esther Sammler
Abstract
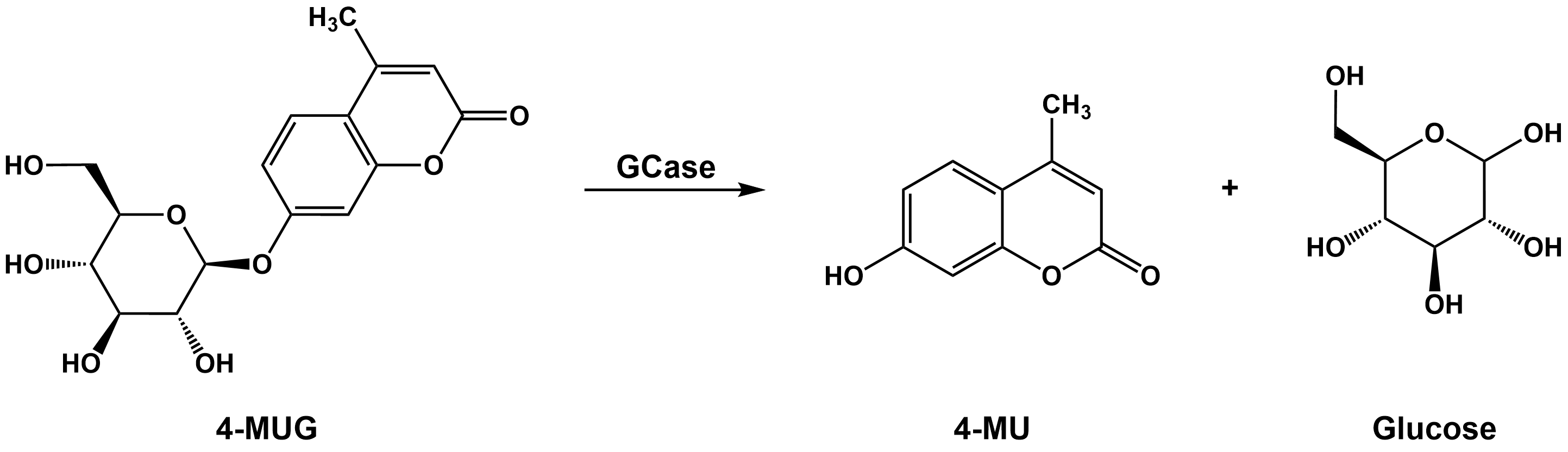
Here we report a method to measure enzyme activity of lysosomal glucocerebrosidase (GBA1, GCase) by monitoring the hydrolysis of the fluorescent substrate 4-methylumbelliferyl-β-D-glucopyranoside. The assay is performed at low pH, at which non-lysosomal glucocerebrosidase activity is expected to be low. This is consistent with the abolishment of 4-MUG hydrolysis in the presence of the GBA1 inhibitor CBE. Our data shows that GBA1 activity is significantly increased in purified lysosomes compared to the whole cell extract.
Steps
Buffer preparation
0.1M citric acid: dissolve 19.2g in 1L
0.2M sodium phosphate: dissolve 28.4g in1L.
Citrate-phosphate buffer, pH 5.4: mix 44.2mLwith 56.8mL to make 100mL citrate-phosphate buffer, 5.4.
0.5M EDTA: dissolve 20.8g in 80mL Adjust to 8and top up to 100mL.
Assay buffer: to make 500 mL assay buffer, add 1.25g , 5g, and 1mL to 500mL.
Stop buffer: dissolve 37.5g in 400mL. Adjust to 12.5 and top-up volume to 500mL.
10mM 4-MU calibrator stock solution: dissolve 17.6mg in 10mL. Aliquot and store at -20 °C, protected from light.
25 mM CBE: dissolve 5mg in 1.23mL. Aliquot and store at -20°C.
Substrate preparation
Dissolve 4.2mg in 2.5mL (final concentration = 5millimolar (mM)). A sonicator water bath may be used to facilitate dissolution.
Sample preparation
Add 5µg of protein from whole cell extracts or 1µg of protein from Lyso-IP samples into the wells of a flat bottom black 96-well plate in duplicate.
Top up volume to 80µL with assay buffer.
Add 1.2µL of DMSO or 25mM CBE to each sample well.
Prepare blank samples in duplicate: add 80µL to two empty wells.
Prepare calibrator wells: designate 24 empty wells for the calibrators and add 100µL assay buffer to each of these wells.
Enzymatic reaction
Add 20µL of the 5mM 4-MUG solution prepared in step 9 to each of the sample and blank wells.
Cover the plate and incubate at 37°Cfor 1h 0m 0s.
Preparation of calibrator serial dilutions
During the incubation, thaw an aliquot of 10mM 4-MU.
Label 11 1.5mL microcentrifuge tubes with numbers 1-11.
Add 1mL to tube 1.
Add 500µLto tubes 2-11.
Add 2µL to tube 1.
Mix by pipetting up-and-down and transfer 500µL from tube 1 to tube 2.
Repeat step 22 sequentially for the remaining tubes. At the end, only tube 11 should contain 1 mL.
| A | B | C | D |
|---|---|---|---|
| Tube | Volume of Stop buffer (µL) | Volume and source of 4-MU (µL) | Final 4-MU concentration (nM) |
| 1 | 1000 | 2 of 10mM stock | 20 000 |
| 2 | 500 | 500 of tube 1 | 10 000 |
| 3 | 500 | 500 of tube 2 | 5 000 |
| 4 | 500 | 500 of tube 3 | 2 500 |
| 5 | 500 | 500 of tube 4 | 1 250 |
| 6 | 500 | 500 of tube 5 | 625 |
| 7 | 500 | 500 of tube 6 | 312.5 |
| 8 | 500 | 500 of tube 7 | 156.25 |
| 9 | 500 | 500 of tube 8 | 78.12 |
| 10 | 500 | 500 of tube 9 | 39.06 |
| 11 | 500 | 500 of tube 10 | 19.53 |
Calibrator concentrations.
Stop reaction and fluorescence measurement
Add 100µL to each sample and blank well.
Add 100µLto 2 of the calibrator wells. These will be the blanks for the calibration curve.
Add 100µL of each calibrator solution prepared in step 23 to 2 of the calibrator wells.
Measure fluorescence intensity in a plate reader (Ex/Em = 350/460).
Data analysis
Plot the fluorescence intensity of the calibrator against the corresponding amounts
of 4-MU in picomoles (pmol). Determine the linear equation representing this
relationship.
Using the calibration curve equation, estimate the amount of released 4-MU in picomoles for the
samples.