LAMP-QUASR Primer Design
Fernan Federici, Felipe Navarro Martínez
Abstract
This protocol is designed for the design and adaptation of a LAMP primer set in order for it to be used with the QUASR technique. In order to do this, it requires an already designed (and hopefully experimentally validated) LAMP primer set. The steps indicated in this protocol are the ones we follow to adapt a previously validated primer set for their use in LAMP-QUASR reactions.
QUASR is "a simple technique that allows closed-tube, target-specific detection, based on the inclusion of a dye-labeled primer that is incorporated into a target-specific amplicon if the target is present. A short, complementary quencher hybridizes to unincorporated primer upon cooling down at the end of the reaction, thereby quenching the fluorescence of any unincorporated primer" (Ball et al., 2016).

Therefore, using QUASR-LAMP allows for specific sequence detection and reduces the false positives often associated with unspecific amplification, and it only requires a minor modification of one of the LAMP reaction primers.
This protocol mostly focuses on the software and criteria we use to decide which primer of the set should be picked, modified with a fluorophore, and used as a template for quencher probe design. Some steps are based on the criteria used by Priye et al. (2017), with several modifications and additions of our own.
Steps
Analyze existing primer set
In order to choose a primer for QUASR probe design, is important to understand the parameters present in a LAMP primer set.
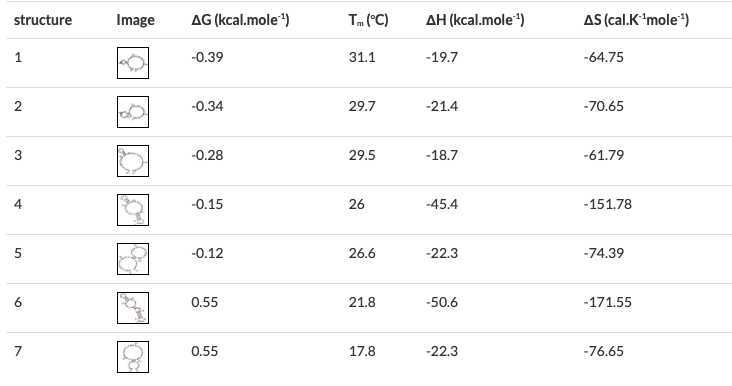
One way to do this, is by checking the complete set of primers (including full-length FIP and BIP) for self-dimerization and/or cross-dimerization using the Multiple Primer Analyzer Multiple Primer Analyzer tool provided by Thermo Fisher and observing the results for primer dimer formation.
The default sensitivity value should be 3, for which few to none dimers should be expected. We recommend changing the sensitivity value to 2 and observe the self-dimers and cross-dimers that form. 2 and observe the self-dimers and cross-dimers that form.
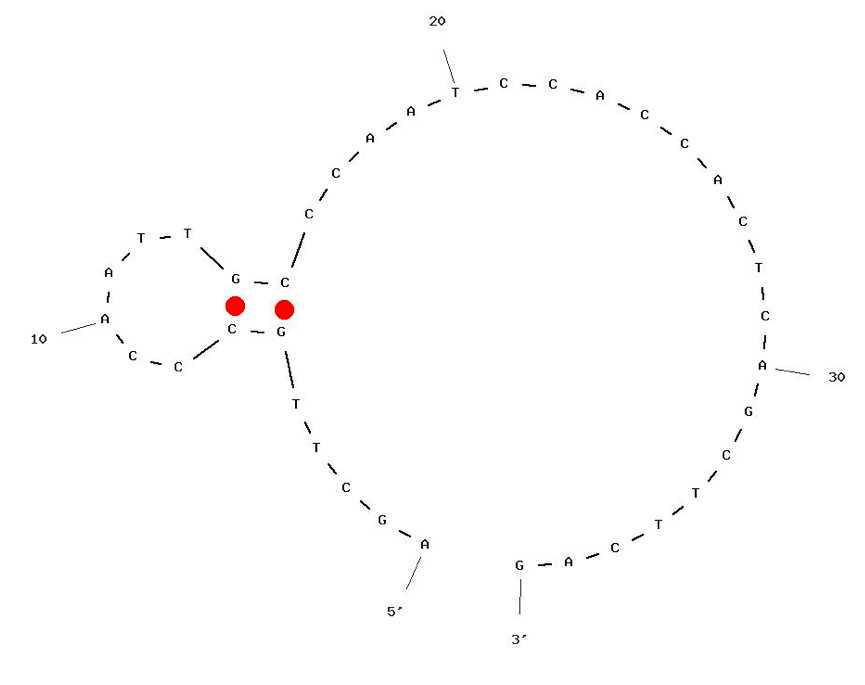
It is also important to check any hairpin structures formed by each primer and analyze them in terms of their stability and location among the sequence.
This can be achieved by analyzing each individual primer using the OligoAnalyzer OligoAnalyzer tool provided by Integrated DNA Technologies , with default settings except adjusting Mg++ concentration to 8 mM , the default for a LAMP reaction.
Choose an individual primer for further QUASR modification. If possible, prefer a FIP or BIP labelled primer, as they are present in a higher concentration in the reaction and therefore produce results with higher contrast.
QUASR Probe: Design and Analysis
Design a quencher probe, by choosing the complementary sequence of the first 10-13 bp of the labelled primer chosen in step 3.
We recommend starting with 13 bp and reduce the length of the probe according to the requirements indicated in the steps below.
Determine the Tm of the quenching probe, which should have a value below 55ºC.
If the designed probe does not fulfill this requirement, and reduce the length of the quenching probe by 1 bp until the correct Tm is achieved. and reduce the length of the quenching probe by 1 bp until the correct Tm is achieved.
Analyze, using Multiple Primer Analyzer , if the addition of the quenching probe to the primer set leads to the the formation of new cross-dimers.
Keep in mind that a very stable dimer between the quenching probe and the fluorescent primer is expected , as this interaction is the basis of how QUASR works.
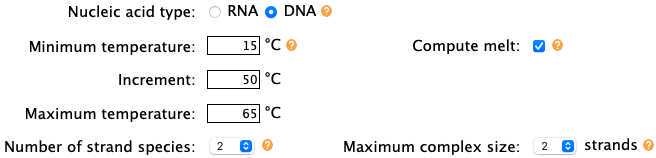
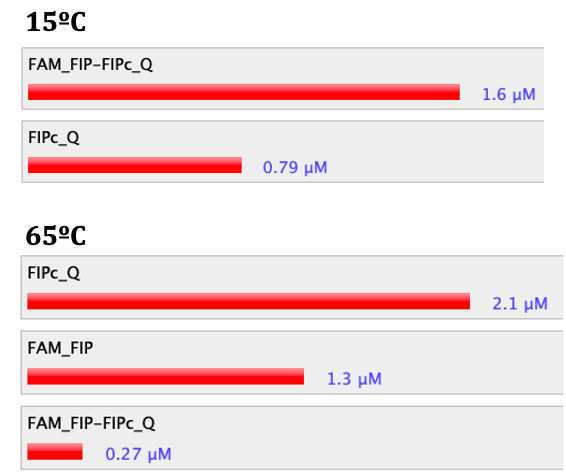
Analyze the interactions between fluorescent primer and quenching probe at different temperatures using NUPACK with the following settings:
Comparison and Selection
If you have a quenching probe of 11-13 bp , reduce its length by one base pair. Then, to see if the change in length of the quenching probe leads to less dimer formation and/or better results in the NUPACK analysis.
This simple step should not be overlooked, as we have seen this small change can reduce the stability of primer dimers and significantly change the proportions observed in the NUPACK analysis, leading to bigger discrimination between positive and negative reactions. should not be overlooked , as we have seen this small change can reduce the stability of primer dimers and significantly change the proportions observed in the NUPACK analysis, leading to bigger discrimination between positive and negative reactions.
If the shortest quenching probe designed still has more than 10 bp, repeat step 8 and compare the results between different probes.
If another primer of the set also seems suitable for QUASR modification, it is recommended to repeat steps 4 to 8 with this primer and compare the results between different possible quenching probes.
Select a suitable QUASR primer based on the criteria indicated in step 2 and the results obtained from the previous analyses.
- If possible, prefer a FIP or BIP labeled primer , as they are present in a higher concentration in the reaction and therefore produce results with higher contrast
- If you plan to test two QUASR primers, we recommend trying out with one FIP or BIP-labeled primer and another LF or LB-labeled primer
Order the fluorescent-labelled primer and its corresponding quenching probe, and test them experimentally in your LAMP-QUASR reactions.