Increasing insulin measurement throughput by fluorescence anisotropy imaging immunoassays
Michael Roper, Damilola I. Adeoye, Yao Wang, Yue J. Wang
blood glucose
insulin secretion
fluorescence anisotropy
immunoassays
polarizers
Islets of Langerhans
Hormone
Abstract
Insulin secreted from islets of Langerhans is the main hormone to reduce blood glucose. Examination of insulin secretion patterns at the single islet level reveals functional differences in the timings and patterns of release. This heterogeneous response highlights the importance of developing systems to measure dynamic release from small numbers of islets in parallel. Toward this, we describe fluorescence anisotropy imaging immunoassays as a relatively simple method for increased throughput of islet secretion measurements. In this system, vacuum pressure from a syringe pump pulled perfusate from 12 islet chambers and reagents into 12 parallel mixing channels for a competitive immunoassay. Light from a Xe arc lamp was filtered and polarized prior to focusing on the microfluidic device at the region where the 12 mixing channels converged. Emission was collected and passed through vertical and horizontal emission polarizers housed in an automated filter wheel before being imaged with a sCMOS camera for the determination of anisotropy. This microfluidic system was tested by monitoring insulin release from groups of murine and human islets. Heterogeneity was observed in the islet traces; however, the presence of islets affected the resistance of the islet chambers, hampering insulin quantification. Nonetheless, this microfluidic system is a step towards increasing the throughput of hormone release measurements from islets of Langerhan.
Graphical abstract

Steps
Experimental
Chemicals and reagents
Sodium chloride, calcium chloride, sodium hydroxide, ethylenediaminetetraacetic acid (EDTA), Tween-20, and bovine serum albumin (BSA) were from EMD Chemicals (San Diego, CA). Dextrose, RPMI 1640, gentamicin, and fetal bovine serum were from Thermo Fisher Scientific (Waltham, MA). Collagenase P (from Clostrdium histolyticum) was acquired from Roche Diagnostics (Indianapolis, IN). Monoclonal insulin antibody (Ab) was purchased from Meridian Life Science, Inc. (Saco, ME). Fluorescein isothiocyanate labeled insulin (insulin*) and other reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless noted otherwise. All solutions were made with Milli-Q (Millipore, Bedford, MA) 18 MΩ cm ultrapure water and filtered using 0.2 μm nylon syringe filters (Pall Corporation, Port Washington, NY).
Immunoassay reagents (insulin* and Ab) were prepared in TEAT-40 (7.4) composed of 25 mM tricine, 40 mM NaCl, 1 mM EDTA, 0.1% Tween-20 (w/v), and 1 mg mL-¹ BSA. 200 nM of the insulin* and Ab were placed in their respective reservoirs for experiments. Islets or insulin standards were placed in a balanced salt solution (BSS) (7.4) that consisted of 125 mM NaCl, 2.4 mM CaCl², 1.2 mM MgCl², 5.9 mM KCl, 25 mM tricine, 1 mg mL-¹ BSA, and the appropriate glucose concentration as described in the text.
Microfluidic device and system
The microfluidic device was fabricated in borosilicate glass (Telic Company, Santa Clarita, CA) using wet chemical etching techniques previously described [15,26]. Microfluidic channels were 50 μm deep and 75 μm wide in the middle measured by an SJ-410 surface profiler (Mitutoyo Corp., Aurora, IL). Fluidic access holes for immunoassay reagents and islet chambers were drilled using 0.02′′ and 0.012′′ diamond-tipped drill bits (Industrial Power Tool and Abrasives, NY), respectively. Fluidic reservoirs (IDEX Health and Science, Oak Harbor, WA) were bonded to the microfluidic device according to the manufacturer’s instructions. A syringe pump (Harvard Apparatus, Holliston, MA) was connected to the common outlet on the device with Tygon tubing (0.02′′ ID × 0.06′′ OD, Cole-Parmer North America, Vernon Hills, IL) through a fingertight fitting (IDEX Health and Science).
Optical detection system
The microfluidic device was placed on a motorized XY microscope stage (Zaber Technologies Inc., Vancouver, British Columbia, Canada) mounted on a Nikon Eclipse Ti–S inverted microscope (Nikon Instruments Inc., Melville, NY). Excitation light from a Xenon arc lamp (Lambda XL, Sutter Instruments, Novato, CA) was sent through a 485 ± 35 nm bandpass filter (Semrock, Rochester, NY) and coupled to the microscope with a liquid light guide. The light then passed a linear polarizer (WP25M-VIS, Thorlabs Inc., Newton, NJ) and was reflected by a dichroic mirror (FF506-Di02-25 × 36, Semrock) and focused onto the microfluidic device using a 10 × 0.5 NA objective (Nikon). Fluorescence emission was collected by the same objective and filtered using a 536 ± 40 nm bandpass (Semrock) emission filter. The emission was sent through an automated emission filter wheel (Applied Scientific Instrumentation, Eugene, OR) that contained linear polarizers, oriented parallel and perpendicular with respect to the excitation polarizer, and imaged with a sCMOS camera (Prime BSI express, Photometrics, Tucson, AZ). Each image was acquired with a 10 s exposure time. Micro-manager software [30,31] was used to control image capture, filter wheel, and the XY microscope stage movement. A microscope environmental chamber (World Precision Instruments, Sarasota, FL) was used to maintain the device and solutions for all experiments at 37°C unless otherwise noted.
Isolation and culture islets of Langerhans
Murine pancreatic islets were isolated from CD-1 male mice (Charles River Laboratories, Wilmington, MA) according to the Florida State University Animal Care and Use Committee (Protocol number 202000078) as previously described [32]. Isolated islets were cultured in RPMI 1640 with 10% fetal bovine serum, 100 U mL-¹penicillin, 100 μg mL-¹streptomycin, and 10 μg mL-¹ gentamycin (Eppendorf North America, Enfield, CT) at 37°C and 5% CO². Islets were used within 4 days after isolation.
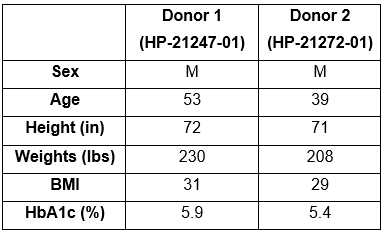
Human islets, obtained from Prodo Laboratories (Aliso Viejo, CA), were from deidentified cadaveric organ donors and, therefore, exempt from Institutional Review Board approval. The donor characteristics are given in Table S1 . Human islets were cultured at 37°C and 5% CO²in PIM(S) islet-specific media (Prodo Laboratories).
Prior to each experiment, islets were washed in a dish of prewarmed BSS containing 3 mM glucose for 10 min. Groups of five murine islets or seven human islets were then loaded into each of the 12 islet chambers on the microfluidic device.
Data analysis
The average fluorescence intensity from each parallel and perpendicular image pair was measured from a region of interest (ROI) in ImageJ [33] and converted to anisotropy using Equation (1) . Each point on the calibration curves is the average anisotropy from 5 consecutive measurements with error bars showing ±1 standard deviation (SD). Calibrations plots were fitted with a four-parameter logistic function using a MATLAB (MathWorks, Natick, MA) script written in-house. The limit of detection (LOD) was taken as the concentration of insulin that decreased the anisotropy value of the blank solution by 3 times the SD. For presentation, islet traces were smoothed using the built-in smoothing ‘rloess’ function in MATLAB with a span of 7 points, which can be called by smooth(y, ’rloess’). This smoothing function fits a weighted least squares 2nd degree polynomial to the span of data.

