High-Content Screening of Synaptic Density Modulators in Primary Neuronal Cultures
Audrey Coulon, Audrey Coulon, Dolores Siedlecki-Wullich, Dolores Siedlecki-Wullich, Chloé Najdek, Chloé Najdek, Carla Gelle, Carla Gelle, Anne-Marie Ayral, Anne-Marie Ayral, Florie Demiautte, Florie Demiautte, Erwan Lambert, Erwan Lambert, Alexandre Vandeputte, Alexandre Vandeputte, Priscille Brodin, Priscille Brodin, Tiago Mendes, Tiago Mendes, Jean-Charles Lambert, Devrim Kilinc, Julie Dumont, Julien Chapuis
Abstract
The synapse, which represents the structural and functional basis of neuronal communication, is one of the first elements affected in several neurodegenerative diseases. To better understand the potential role of gene expression in synapse loss, we developed an original high-content screening (HCS) model capable of quantitatively assessing the impact of gene silencing on synaptic density. Our approach is based on a model of primary neuronal cultures (PNCs) from the neonatal rat hippocampus, whose mature synapses are visualized by the relative localization of the presynaptic protein Synaptophysin with the postsynaptic protein Homer1. The heterogeneity of PNCs and the small sizes of the synaptic structures pose technical challenges associated with the level of automation necessary for HCS studies. We overcame these technical challenges, automated the processes of image analysis and data analysis, and carried out tests under real-world conditions to demonstrate the robustness of the model developed. In this article, we describe the screening of a custom library of 198 shRNAs in PNCs in the 384-well plate format. © 2023 The Authors. Current Protocols published by Wiley Periodicals LLC.
Basic Protocol 1 : Culture of primary hippocampal rat neurons in 384-well plates
Basic Protocol 2 : Lentiviral shRNA transduction of primary neuronal culture in 384-well plates
Basic Protocol 3 : Immunostaining of the neuronal network and synaptic markers in 384-well plates
Basic Protocol 4 : Image acquisition using a high-throughput reader
Basic Protocol 5 : Image segmentation and analysis
Basic Protocol 6 : Synaptic density analysis
INTRODUCTION
Failure to correctly regulate the number and distribution of synapses is a neuropathological feature shared by a variety of neurological and psychiatric disorders, such as autism (Monteiro & Feng, 2017), epilepsy (Wong & Guo, 2013), schizophrenia (Gigg et al., 2020), amyotrophic lateral sclerosis (Fogarty, 2019), Parkinson's disease (Bellucci et al., 2016), and Alzheimer's disease (AD) (DeKosky & Scheff, 1990). Identifying chemical or biological modulators of synaptic density may contribute to the understanding of pathophysiological mechanisms and to the identification of new therapeutic targets. Thus, our objective was to develop a medium-throughput assay to assess the impact of each currently known AD genetic risk factor (Bellenguez et al., 2022) on the synaptic density of primary neuronal cultures (PNCs).
In this context, high-content screening (HCS) of a library of short-hairpin RNAs (shRNAs) that individually target each of these genetic risk factors provides a powerful approach to systematically analyze their impact on synaptic density. Compared to high-throughput screening (HTS), which evaluates the activity of thousands of compounds in parallel in miniaturized biochemical or conventional cellular assays, HCS provides more biologically complex information through the use of fluorescence microscopy, multiplexing, and sophisticated image analysis at the cellular or sub-cellular scale. HCS can therefore be useful for studying diseases where the disease-associated cellular phenotypes are known but the underlying molecular mechanisms are not fully characterized, such as AD. To understand the implications of a quantitative analysis of synaptic density using HCS, several critical points should be considered. First, although the resolution of HCS/HTS microscopes has been strongly improved over the last decade, visualization of small structures such as synapses (<100 nm) requires one to work with the highest magnification available. Another important consideration is that the detection of mature synapses has been strongly improved by analyzing the colocalization of a presynaptic marker and a postsynaptic marker (Nieland et al., 2014; Verschuuren et al., 2019), even though this makes the image acquisition and analysis cumbersome. Second, these automated approaches require a robust and reproducible assay to overcome additional limitations, such as the complexity of the neuronal network and the heterogeneity in synaptic density in primary cultures. Third, the choice of synaptic markers used is also a critical point because antibodies may show non-uniform labeling (Verstraelen et al., 2020). Last, the majority of HCS methodologies aiming to visualize synapses are usually developed in the 96-well plate format, which limits screening capacity and/or increases the associated costs.
We recently developed an image analysis workflow that permits us to identify synapses by the proximity-based assignment of postsynaptic puncta to presynaptic puncta (Kilinc et al., 2020). The workflow is based on the detection of fluorescent puncta following immunolabeling of the endogenous synaptic proteins Synaptophysin and Homer1, and the automated analysis of their relative localization. Here, we adapted this workflow to the 384-well plate format and used it in the HCS of a lentiviral shRNA library targeting 198 AD-associated genes (Bellenguez et al., 2022). The screen was organized in two parts: (i) the 105 genes we reported in 2019 (Kunkle et al., 2019) and (ii) the 93 genes we reported in 2022 (Bellenguez et al., 2022). The assay is reproducible and robust and therefore constitutes a promising medium-throughput screening tool to identify new compounds of potential therapeutic interest in neurodevelopmental and neurodegenerative diseases.
In this article, we describe (i) the culture of primary hippocampal neurons in 384-well plates (Basic Protocol 1), (ii) their transduction with lentiviral shRNAs (Basic Protocol 2), (iii) the immunolabeling of synaptic and structural markers (Basic Protocol 3), (iv) image acquisition using a high-throughput reader (Basic Protocol 4), (v) the automated image analysis process (Basic Protocol 5), and (vi) synapse density quantification (Basic Protocol 6).
Basic Protocol 1: CULTURE OF PRIMARY HIPPOCAMPAL RAT NEURONS IN 384-WELL PLATES
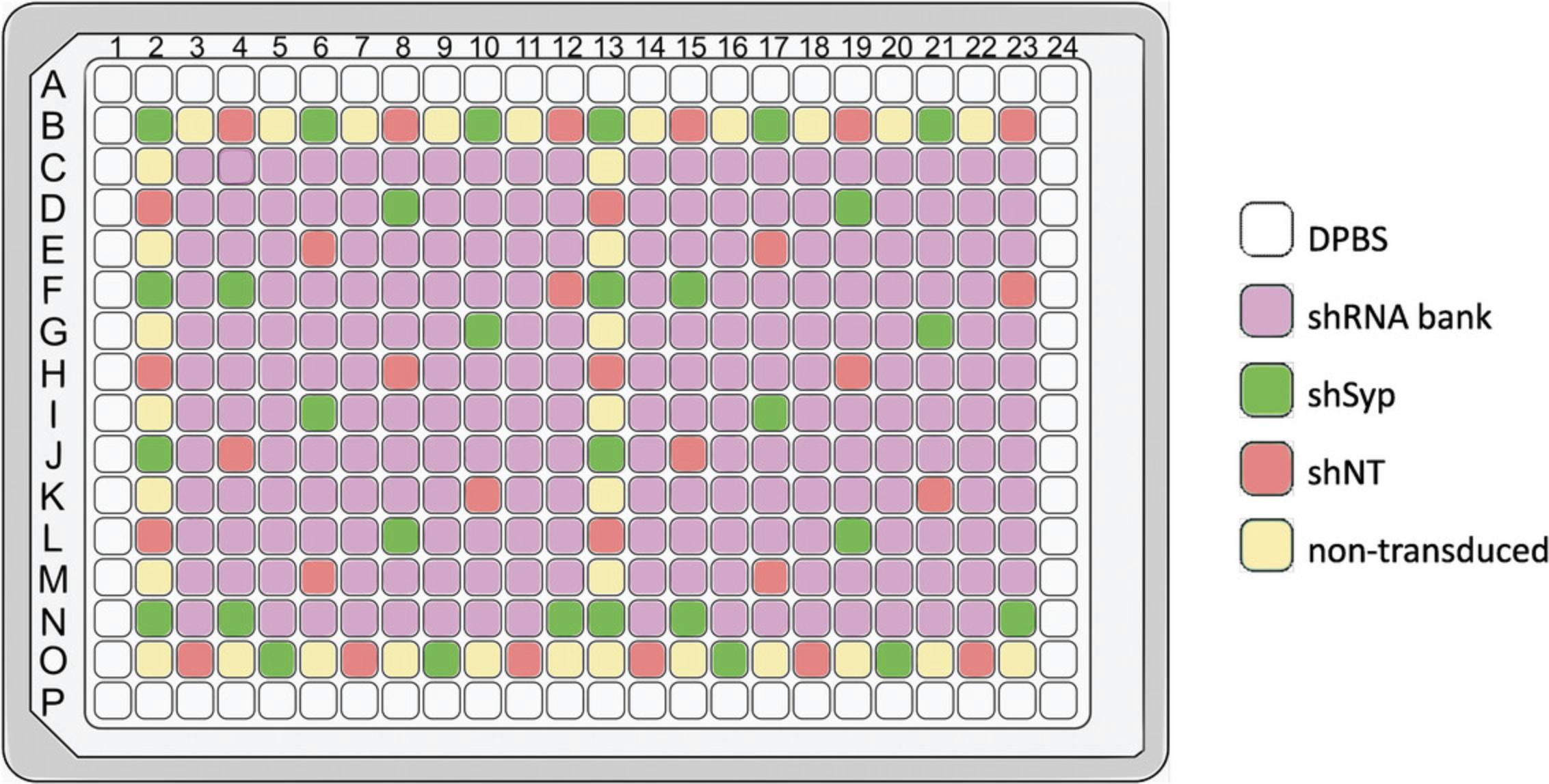
This protocol is adapted from a classical protocol of primary hippocampal neuron culture (Kaech & Banker, 2006; see Current Protocols article: Mendes et al., 2020). The aim of HCS is to study the effect of a large number of compounds or genes in a limited time. To increase experimental efficiency, the cell culture needs to be miniaturized. Cells are grown in 384-well plates with a surface area of 8 mm² per well. To enable individual cells to be imaged and analyzed, cell density must be relatively low, cell culture must be homogeneous throughout the well, and cells must not form aggregates. A frequent problem with HCS is the edge effect, i.e., a phenotypic difference between cells seeded in the center of the plate and those seeded at the edge, which can lead to high inter-well variability. To avoid this problem, we chose not to seed cells in the wells at the edge of the plate, but to fill these wells with Dulbecco's phosphate-buffered saline (DPBS). These cultures are maintained for up to 21 days in vitro to ensure sufficient maturation for the formation of a dense neuronal network and functional synapses. The screening of up to 107 lentiviral shRNAs, at two different multiplicity of infection (MOI) values, with each shRNA present in technical quadruplicate, requires the preparation of four 384-well plates with primary hippocampal neurons plated in 308 wells (Fig. 1).
Materials
-
10 µg/ml poly-d-lysine (PDL; Sigma, cat. no. P6407) dissolved in 0.1 M borate buffer (see recipe)
-
1× DPBS
-
P0 Wistar rat pups
-
Dissection buffer (see recipe), 4°C
-
2.5% (w/v) trypsin (Gibco, cat. no. 15090-046), 37°C
-
5 mg/ml DNase I (Sigma, cat. no. DN25; diluted in water)
-
Dissociation medium (see recipe), 37°C
-
Culture medium (see recipe), 37°C
-
Trypan blue
-
384-well cell culture microplates (Greiner Bio-One, cat. no. 781091)
-
16-channel pipet (Finnpipette F1, Thermo Scientific, cat. no. 4661090N)
-
Surgical scissors (Fine Science Tools, cat. no. 14007-14)
-
15- and 50-ml tubes
-
Petri dishes
-
Forceps (Dumont #5; Fine Science Tools, cat. no. 11254-20)
-
Inverted binocular microscope
-
Agitator (Fisher Scientific, cat. no. 13490577) or similar
-
Standard tabletop centrifuge
-
Counting chamber
-
50-ml sterile reservoirs (Clearline, cat. no. 097803)
NOTE : All protocols involving animals must be reviewed and approved by the appropriate Animal Care and Use Committee and must follow regulations for the care and use of laboratory animals.
NOTE : All solutions and equipment coming into contact with cells must be sterile, and proper sterile technique should be used accordingly.
NOTE : All culture incubations are performed in a 37°C, 5% CO2 tissue culture incubator unless otherwise specified.
Coat 384-well plates
1.One day before primary culture, coat 384-well cell culture microplates (excluding edge wells: rows A and P, columns 1 and 24) with 15 μl PDL per well and incubate overnight at 37°C and 5% CO2. Fill edge wells with 100 μl of 1× DPBS.
2.On the day of primary culture, wash the plates twice with 40 µl of 1× DPBS per well using a 16-channel pipet and then keep them with 1× DPBS in the incubator.
Dissect hippocampi
3.Put the P0 Wistar rat pups on ice. Decapitate the pups with surgical scissors and keep the heads in a 50-ml tube containing ice-cold dissection buffer.
4.Transfer a head into a Petri dish filled with ice-cold dissection buffer. With a pair of forceps, pinch the skin at the front of the head and pull it to the back of the head. Open the skull from the back to the front of the head with a pair of forceps and carefully lift the brain with another pair of forceps to transfer it into a second Petri dish filled with ice-cold dissection buffer.
5.Repeat with each of the heads, dispatching the brains into different dishes.
6.Put one of the dishes under an inverted binocular microscope at 10× magnification and separate the two hemispheres of the brain. Lay each hemisphere with its ventral side up and pinch the olfactory bulb backward to pull out the meninges. Flip the hemisphere with its ventral side down and carefully peel the meninges off.
7.Dissect the hippocampus from each cortex and transfer to a 15-ml tube fully filled with ice-cold dissection buffer.
8.Repeat steps 6 and 7 for each brain before proceeding to the next step.
Dissociate and plate neurons
9.Wash the hippocampi three times with 10 ml ice-cold dissection buffer.
10.Remove all the dissection buffer and add 0.25 ml pre-warmed 2.5% trypsin per brain. Incubate for 10 min on an agitator or similar placed in the tissue culture incubator.
11.During the incubation, add 12.5 µl of 5 mg/ml DNase I in 10 ml pre-warmed dissociation medium per brain.
12.Remove as much trypsin as possible using a 1000-µl pipet tip and then add the 10 ml of dissociation medium with DNase. Invert each tube three times, letting the hippocampi sediment between inversions.
13.Wash the hippocampi three times with 10 ml pre-warmed dissociation medium.
14.Remove all the dissociation medium and add 2 ml pre-warmed culture medium. Mechanically dissociate the tissue using a 1000-µl pipet tip by gently pipetting up and down 30 times.
15.Centrifuge 8 min at 200 × g at room temperature.
16.Remove the supernatant and resuspend the cells in 2 ml pre-warmed culture medium.
17.Dilute 10 μl cell suspension in 40 μl pre-warmed culture medium and 50 μl trypan blue.
18.Count the cells using a counting chamber.
19.Dilute the cell suspension in pre-warmed culture medium to 100,000 cells/ml.
20.Pour the cell suspension into a 50-ml sterile reservoir and add 40 µl to each well within rows B to O and columns 2 to 23 using a 16-channel pipet.
21.Incubate the screening plates overnight in the tissue culture incubator and proceed directly to Basic Protocol 2.
Basic Protocol 2: LENTIVIRAL shRNA OF PRIMARY NEURONAL CULTURE IN 384-WELL PLATES
The choice of experimental controls is crucial in HCS, as controls help to ensure data quality and reliability. Controls should be chosen to produce an easily detectable and reproducible response. As a positive control, we choose to use an shRNA targeting Synaptophysin (shSyp), resulting in a decrease in the expression of the corresponding protein. The negative control used is a non-targeting shRNA (shNT). The transduction is performed at 1 day in vitro (DIV1) according to the plate map (Fig. 1). Each shRNA is present in technical duplicates in each plate, and two plates are transduced for each MOI, namely MOI2 and MOI4.These numbers are arbitrary and can be adapted to other HCS applications. Our plate map allows for testing the impact of the under-expression of a maximum of 107 genes of interest in technical quadruplicates at two different MOIs.
Materials
- 1000× polybrene (Hexadimethrine bromide 4 mg/ml; Sigma, cat. no. 107689-10G)
- Culture medium (see recipe)
- Four screening plates, containing rat postnatal hippocampal cultures at DIV1 (see Basic Protocol 1)
- Lentiviral shRNA bank source plate, containing lentiviral shRNAs targeting genes of interest and lentiviral control shRNAs shNT (Merck, MISSION® shC002V) and shSyp (Merck, MISSION® NM_009305 TRCN0000379864)
- 384-well plates (for intermediate dilutions; Brand, cat. no. 701355)
- 16-channel pipet (Finnpipette F1; Thermo Scientific, cat. no. 4661090N)
NOTE : Use sterile pipet tips with filters (Finntip 50; Thermo Scientific, cat. no. 94052060).
NOTE : All culture incubations are performed in a 37°C, 5% CO2 tissue culture incubator unless otherwise specified.
Change medium in the screening plates
1.Dilute 6.7 µl of 1000× polybrene in 10 ml culture medium (0.67× polybrene) and warm it to 37°C.
2.Warm culture medium to 37°C.
3.Allocate two screening plates each for transduction at MOI2 and MOI4 (four plates total).
4.For each MOI4 screening plate, remove 40 µl medium from each well and add 10 µl fresh pre-warmed culture medium from step 2.
5.For each MOI2 screening plate, remove 40 µl medium from each well and add 15 µl fresh pre-warmed culture medium containing 0.67× polybrene from step 1.
6.Store the screening plates in the incubator until the intermediate plates are ready (see steps 7 to 11).
Prepare intermediate plates
7.To dilute the lentiviruses from the source plate into the intermediate plate to reach a transduction unit of 1.6 × 106 viral particles per milliliter (VP/ml), calculate the volume of the medium required separately for each shRNA in the bank (Fig. 2).
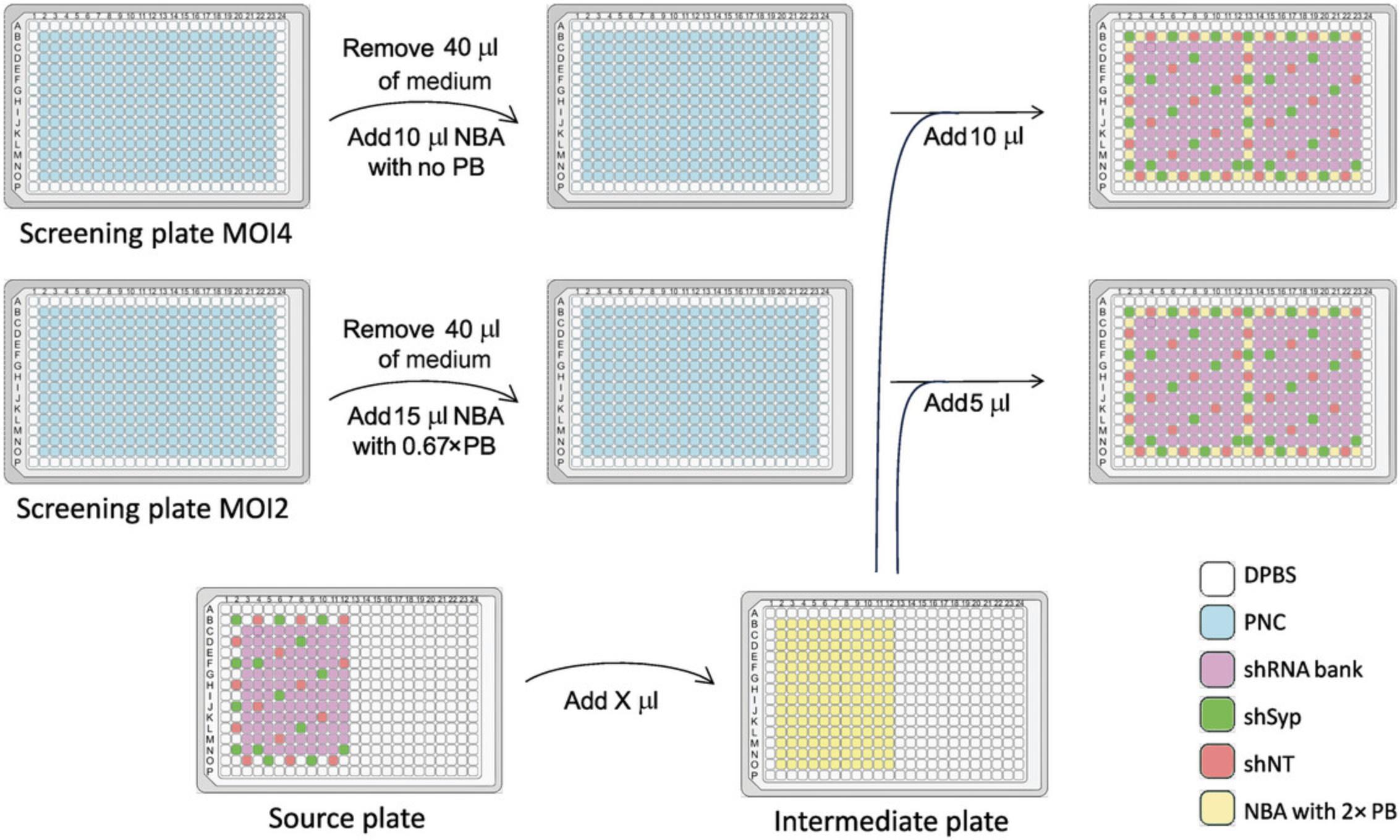
8.For each shRNA, round the volume of medium required to dilute the lentivirus suspension (equal to the final volume calculated in step 7 minus the volume of the lentiviral suspension) to the nearest 10 µl.
9.Thaw the lentiviral shRNA bank source plate.
10.Dilute 40 µl of 1000× polybrene in 20 ml pre-warmed culture medium to obtain 2× polybrene. Using a single-channel pipet, fill the wells of a 384-well plate (the intermediate plate) with the different volumes of medium calculated in step 7 for each shRNA. Fill the non-transduced wells with 100 µl pre-warmed culture medium.
11.Using a 16-channel pipet, transfer the volume of shRNAs from the source plate to the corresponding wells of the intermediate plate.
Transduce cells with lentiviruses
12.Transfer 10 µl lentivirus suspension at 1.6 × 106 VP/ml from each well of the intermediate plate to the corresponding well and its duplicate in the two MOI4 screening plates (see step 6).
13.Transfer 5 µl lentivirus suspension at 1.6 × 106 VP/ml from each well of the intermediate plate to the corresponding well and its duplicate in the two MOI2 screening plates (see step 6).
14.Incubate screening plates for 6 hr in the tissue culture incubator.
15.Add 20 µl fresh pre-warmed NBA medium to each transduction well (40 ml in total).
16.Incubate the screening plates in the tissue culture incubator until DIV21 and then proceed directly to Basic Protocol 3.
Basic Protocol 3: IMMUNOSTAINING OF THE NEURONAL NETWORK AND SYNAPTIC MARKERS IN 384-WELL PLATES
This HCS approach is based on the immunolabeling of a presynaptic protein (Synaptophysin) and a postsynaptic protein (Homer1) and the quantitative analysis of their relative positions. This strategy has recently been shown to have sufficient sensitivity to detect the changes in synaptic connectivity induced by exposing neurons to cell-secreted Aβ oligomers at physiologically relevant concentrations (Kilinc et al., 2020). To ensure an efficient and reproducible immunolabeling process in 384-well plates, washing steps are automated using a robotic platform. To reduce the damage to fixed cells, the position of the distribution/aspiration heads is optimized and the flow rate is reduced. Automated steps of the protocol need to be adapted to the material available to the user. Addition of primary and secondary antibody mixes, however, is done manually to avoid waste due to the relatively large dead volume.
Materials
- Four screening plates, containing transduced rat hippocampal cultures at DIV21 (see Basic Protocol 2)
- 4% (v/v) formaldehyde in DPBS
- 0.3% (v/v) Triton X-100 in DPBS
- 2.5% (w/v) bovine serum albumin (BSA) in DPBS with 0.1% (v/v) Triton X-100
- Primary antibodies:
- Chicken anti-Homer1 (Synaptic Systems, cat. no. 160006)
- Guinea pig anti-Synaptophysin (Synaptic Systems, cat. no. 101004)
- Mouse anti-MAP2 (Synaptic Systems, cat. no. 188011)
- 1× DPBS
- Secondary antibodies:
- Donkey anti-chicken Alexa 488 (Jackson ImmunoResearch, cat. no. 703-545-155)
- Goat anti-guinea pig Alexa 555 (Life Technologies, cat. no. A21435)
- Donkey anti-mouse Alexa 647 (Jackson ImmunoResearch, cat. no. 715-605-151)
-
- 16-channel pipet (Finnpipette F1; Thermo Scientific, cat. no. 4661090N)
- Aluminum sealing tape (Dutscher, cat. no. 106570, or similar)
- PlateHUB rotating plate-storage carousel (Agilent, model G5500-23447)
- Plate washer associated with liquid handler (BioTek, model EL406)
- Wash_Primary.pro and Wash_Secondary.pro programs (see Supporting Information)
- VWorks automation control software (Agilent)
- Aspirate.LHC and Aspirate-Dispense.LHC subroutines (see Supporting Information)
- Direct Drive Robot (Agilent)
NOTE : Use sterile pipet tips with filters (Finntip 50; Thermo Scientific, cat. no. 94052060).
Fix, permeabilize, and saturate the cells
1.Using a 16-channel pipet, carefully remove the culture medium from each of the four screening plates and add 20 µl of 4% formaldehyde in DPBS per well. Incubate for 15 to 20 min at room temperature.
2.Remove formaldehyde and wash for 10 min with 40 µl DPBS per well.
3.Remove DPBS and permeabilize the cells using 20 µl of 0.3% Triton X-100 in DPBS per well for 5 min.
4.Remove Triton X-100 and saturate the cells using 20 µl of 2.5% BSA in DPBS with 0.1% Triton X-100 per well.
5.Incubate 2 hr at room temperature.
Incubate screening plates with primary antibodies
6.Prepare the primary antibody mix by diluting primary antibodies 1:500 in 1× DPBS.
7.Remove buffer from step 4 and add 15 µl primary antibody mix to each per well.
8.Seal all plates with aluminum sealing tape and incubate overnight at 4°C.
9.Bring assay plates from 4°C to room temperature, remove their seals, and place them on PlateHUB cassette 1 of the PlateHUB rotating plate-storage carousel.
10.Plug a bottle of 1× DPBS bottle to the plate washer associated with the liquid handler and install the distribution heads.
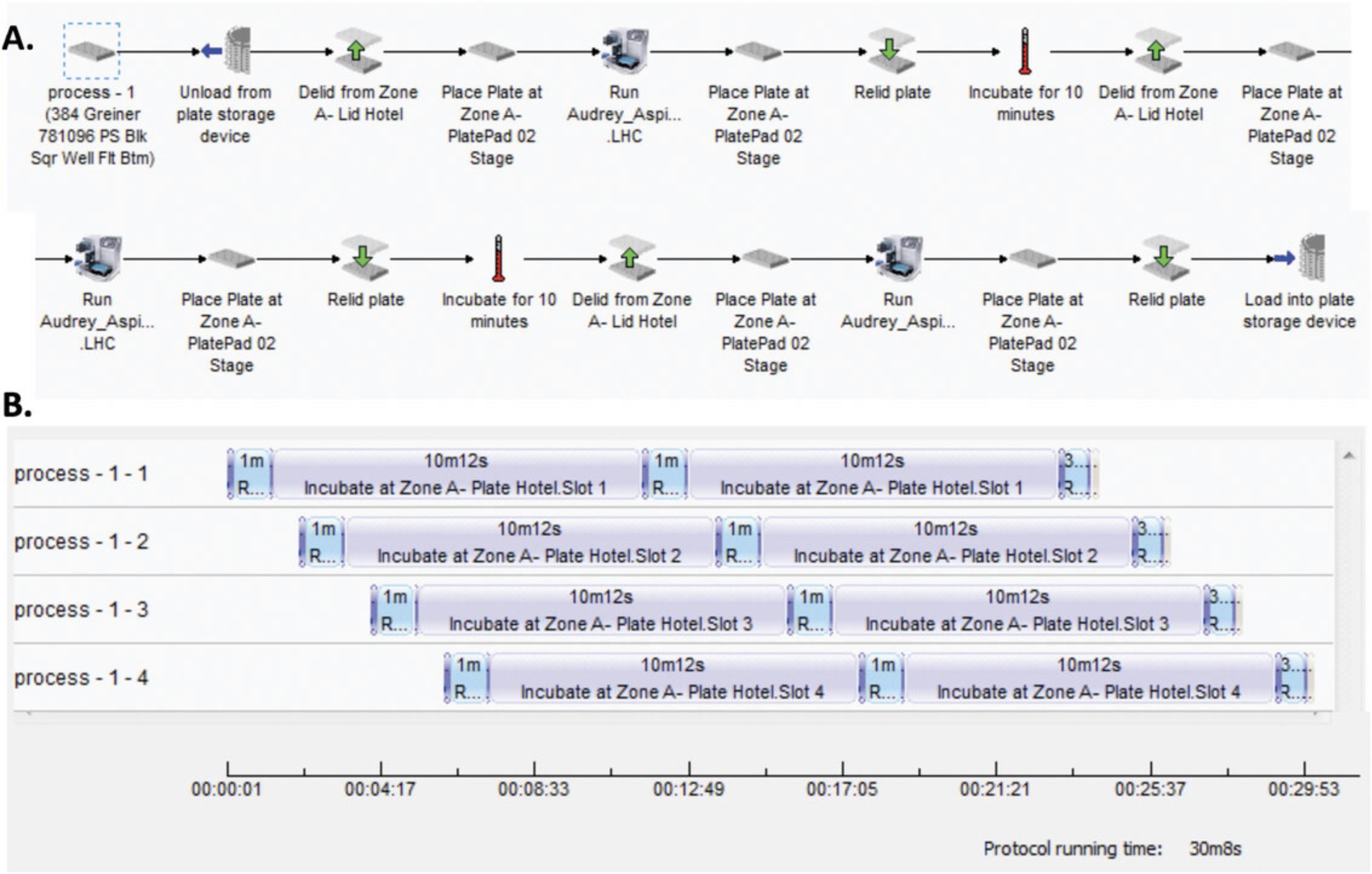
11.Run the program Wash_Primary.pro on the VWorks automation control software by defining the number of plates (four in our case), which allows automated plate transfers by the Direct Drive Robot:
-
Pick a plate, remove its lid, and place it on plate washer.
-
Aspirate the buffer from each well and add 40 μl DPBS per well.
-
Remove the plate from plate washer, replace its lid, and move it to plate pad. Incubate for 10 min.
-
Repeat steps 11a to 11c. During incubation, prepare the secondary antibody mix by diluting secondary antibodies 1:500 in 1× DPBS.
-
Pick a plate, remove its lid, and place it on plate washer.
-
Aspirate the buffer from each well.
-
Remove the plate from plate washer, replace its lid, and move it to PlateHUB cassette 7.
Incubate screening plates with secondary antibodies
12.Each time a plate is placed on PlateHUB cassette 7, move it to the bench and add 15 μl secondary antibody mix per well using a 16-channel pipet.
13.Seal all plates with aluminum sealing tape and incubate 2 hr at room temperature.
14.Run program Wash_Secondary.pro on VWorks by defining the number of plates (four in our case):
-
Repeat steps 11a to 11c twice.
-
Pick a plate, remove its lid, and place it on plate washer.
-
Aspirate the buffer from each well and add 40 μl DPBS per well.
-
Remove the plate from plate washer, replace its lid, and move it to PlateHUB cassette 7.
15.Remove DPBS bottle from the plate washer and wash tubes, pumps, and distribution heads.
16.Proceed immediately to image acquisition (see Basic Protocol 4) for the first plate and store other plates at 4°C.
Basic Protocol 4: IMAGE ACQUISITION USING A HIGH-THROUGHPUT READER
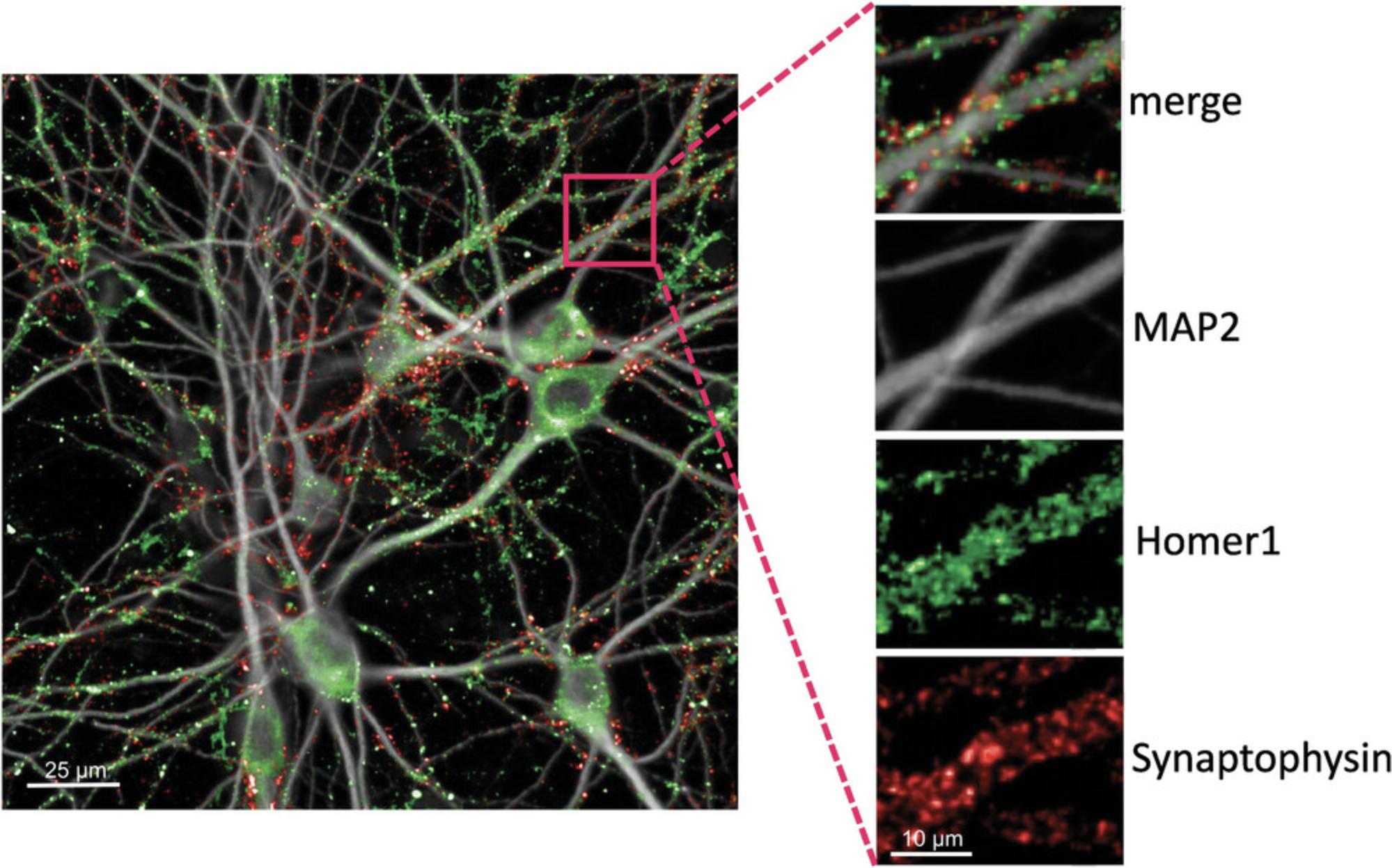
Imaging three wavelengths at sufficiently high spatial resolution (0.1083 μm/px in our case) should produce satisfactory presynaptic and postsynaptic signals, as well as imaging of the somatodendritic network (Fig. 4).
Materials
- Immunostained screening plates (see Basic Protocol 3)
- IN Cell Analyzer 6000 (GE Healthcare; or similar automated confocal microscope)
1.Once immunostaining is completed (see Basic Protocol 3), pick an immunostained screening plate and proceed to setting up the image acquisition parameters (steps 2 to 8).
2.When using the IN Cell Analyzer 6000 (GE Healthcare) for the first time, select (or define) the plate used (in our case, Greiner μclear 384-well plate 781091). Select the lowest-magnification objective available and verify that the autofocus range in different wells of the plate is sufficiently large. Adjust the plate thickness parameter if necessary.
3.In the Dashboard tab, select the highest-magnification objective (in our case, Nikon 60 × /0.95, Plan Apo, Corr Collar 0.11-0.23, CFI/60 Lambda), set binning as 1 × 1, and set laser autofocus power level at 50%. Additionally, select the following acquisition wavelengths: Cy5 (642/706 nm) for MAP2, dsRed (561/605 nm) for Synaptophysin, and FITC (488/525 nm) for Homer1.
4.For all channels, set image mode as “maximum intensity projection” and set Z-slice number as 3.Deselect open aperture and set laser power to 100% and aperture to 1.05.
5.For each channel, define laser intensity as 100% and set up the autofocus offset and exposure time.
6.Define the wells to be imaged: 308 wells from B2 (top left corner) to O23 (bottom right corner).
7.In the Field tab, define number of fields per well as 16, acquisition sequence as “horizontal serpentine”, and spacing between fields as X: 100 µm and Y: 100 µm. Position the fields in the center-right portion of the well.
8.In the Z-Stack setup tab, define the Z Step as 0.5 µm. Position the 3D focus at the center slice.
9.Save image acquisition parameters in a .xaqp file.
10.Insert the first plate in the IN Cell microscope and start automated imaging.
11.Repeat step 10 for the remaining plates. Store imaged plates at 4°C.
12.When the last plate imaged, proceed to Basic Protocol 5 to transfer the images to the Columbus server for image segmentation and analysis.
Basic Protocol 5: IMAGE SEGMENTATION AND ANALYSIS
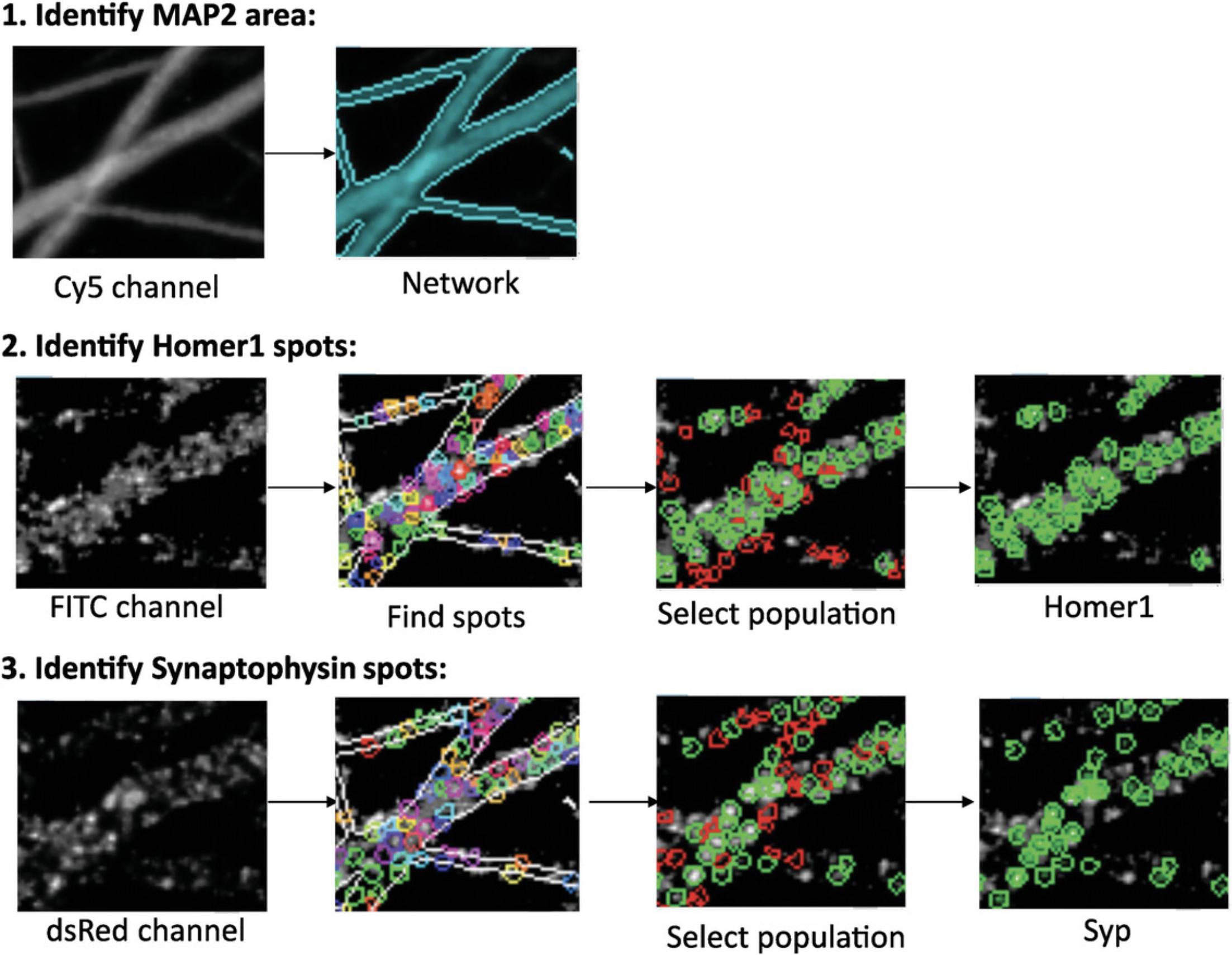
Image segmentation is an essential step for defining the synaptic puncta. Considering the large number of images acquired, this process needs to be done automatically (or semi-automatically) with specialized software. In this protocol, we will describe the image analysis conducted in Columbus software, but the commands can be adapted to any software with similar capabilities (Fig. 5).
Materials
- Columbus Image Data Storage and Analysis System, version 2.7 or above (Perkin Elmer; or similar software)
- Data from Basic Protocol 4
Identify and measure MAP2 areas
1.Open “Import” under the Workflow menu in the Columbus Image Data Storage and Analysis System, select Import Type as “InCell XDCE/TIF”, select source folder (where images and .XDCE files are stored), define the name of the screen/imaging, and start importing data from Basic Protocol 4.
2.Select “Image Analysis”, select an “Assay Plate” under the “Data Tree”, and select an shNT control well from the “Image Selection” menu.
3.In the Input Image building block, select “Maximum Projection” for Stack Processing and “None” for Flatfield Correction.
4.Add a Find Image Region building block with “Channel” = “Cy5”; “ROI” = “None”; “Method” = “Common Threshold”. As second-tier inputs, define “Threshold” as 0.40 and “Area cut-offs” as 0 and deselect “Split into Objects” and “Fill Holes” options. Name “Output Population” and “Output Region” as “Network”.
5.Add a Calculate Morphology Properties building block with “Population” = “Network”; “Region” = “Network”; “Method” = “Standard”. As second-tier inputs, select “Area (in µm²)”. Name “Output Properties” as “Network”.
6.Add a Calculate Intensity Properties building block with “Channel” = “Cy5”; “Population” = “Network”; “Region” = “Network”; “Method” = “Standard”. As second-tier inputs, select “mean” and “sum”. Name “Output Properties” as “Intensity Network”.
Identify and quantify Homer1 spots
7.Add a Find Spots building block with “Channel” = “FITC”; “ROI” = “Network”; “Population” = “Network”; Region = “Network”; “Method” = “C”. As second-tier inputs, set “Radius” to “< 0.1”, “Contrast” to “> 0”, “Uncorrected Spot to Region Intensity” to “> 0”, “Distance” to “≥ 0.75 μm”, and “Spot Peak Radius” to “0.21 μm”. Select “Calculate Spot Properties”. Name “Output Population” as “Homer Spot”.
8.Add a Calculate Intensity Properties building block with “Channel” = “FITC”; “Population” = “Homer Spot”; “Region” = “Homer Spot”; “Method” = “Standard”. As second-tier inputs, select “mean”, “sum”, “max”, “90% quantile fraction”, and “contrast”. Name “Output Properties” as “Intensity Spot FITC”.
9.Add a Select Population building block with “Population” = “Homer Spot”; “Method” = “Linear Classifier”. As second-tier inputs, select “2” as “number of classes” and select the following fields: “Relative Spot Intensity”; “Corrected Spot Intensity”; “Uncorrected Spot Peak Intensity”; “Spot Contrast”; “Spot Background Intensity”; “Spot Area [px2]”; “Region Intensity”; “Spot to Region Intensity”; “Intensity Spot FITC Mean”; “Intensity Spot FITC Maximum”; “Intensity Spot FITC Sum”; “Intensity Spot FITC Quantile 90%”; “Intensity Spot FITC Contrast”. Name “Output Population A” as “Homerselect” and “Output Population B” as “fake”. Choose an shNT control well and click on “Train…”. Select “Class A: green” and click on spots that one could consider as true Homer1 spots. Select “Class B: red” and click on spots that one could consider as falsely identified Homer1 spots. Train the computer by selecting at least 10 spots for each class in at least 10 images from different shNT control wells. Once satisfied with the selection, accept and exit the training.
10.Add another Select Population building block with “Population” = “Homerselect”; “Method” = “Filter By Property”. As second-tier inputs, set “Intensity Spot FITC mean” as “>1500”. Name “Output Population” as “Homer1”.
Identify and quantify Synaptophysin spots
11.Add a Find Spots building block with “Channel” = “dsRed”; “ROI” = “Network”; “Population” = “Network”; Region = “Network”; “Method” = “C”. “Radius” to “< 0.1”, “Contrast” to “> 0”, “Uncorrected Spot to Region Intensity” to “> 0”, “Distance” to “≥ 0.75 μm”, and “Spot Peak Radius” to “0.21 μm”. Select “Calculate Spot Properties”. Name “Output Population” as “Syn Spot”.
12.Add a Calculate Intensity Properties building with “Channel” = “dsRed”; “Population” = “Syn Spot”; “Region” = “Syn Spot”; “Method” = “Standard”. As second-tier inputs, select “mean”, “sum”, “max”, “90% quantile fraction”, and “contrast”. Name “Output Properties” as “Intensity Spot dsRed”.
13.Add a Select Population building block with “Population” = “Syn Spot”; “Method” = “Linear Classifier”. As second-tier inputs, select “2” as “number of classes” and select the following fields: “Relative Spot Intensity”; “Corrected Spot Intensity”; “Uncorrected Spot Peak Intensity”; “Spot Contrast”; “Spot Background Intensity”; “Spot Area [px2]”; “Region Intensity”; “Spot to Region Intensity”; “Intensity Spot dsRed Mean”; “Intensity Spot dsRed Maximum”; “Intensity Spot dsRed Sum”; “Intensity Spot dsRed Quantile 90%”; “Intensity Spot dsRed Contrast”. Name “Output Population A” as “Synselect” and “Output Population B” as “fake(2)”. Choose an shNT control well and click on “Train…”. Select “Class A: green” and click on spots that one could consider as true Synaptophysin spots. Select “Class B: red” and click on spots that one could consider as falsely identified Synaptophysin spots. Train the computer by selecting at least 10 spots for each class in at least 10 images from different shNT control wells. Once satisfied with the selection, accept and exit the training.
14.Add another Select Population building block with “Population” = “Synselect”; “Method” = “Filter By Property”. As second-tier inputs, set “Intensity Spot dsRed mean” as “> 1100”. Name “Output Population” as “Syn1”.
Define Results
15.In the Define Results building block, select “List of Outputs” as “Method”. Open “fake”, “fake(2)”, “Homerselect”, and “Synselect” input fields to confirm that nothing is selected. In “Population: Homer Spots” and “Population: Syn Spots” input fields, select only “Number of Objects”. In “Population: Homer1” and “Population: Syn1” input fields, select “Number of Objects”, set “Apply to all” as “Individual Selection”, and select “ALL” for “Intensity Spot Mean”, “Intensity Spot Maximum”, and “Intensity Spot Sum”. In “Population: Network” input field, deselect “Number of Objects”, set “Apply to all” as “Individual Selection”, and select “ALL” for “Network Area” and “Intensity Network Sum”.
16.For steps 17 to 21, remain in the same Define Results building block, add “Formula” as “Method” and confirm that “a/b” is set as “Formula”.
17.Select “Syn1 - Number of Objects” as “Variable A” and “Network - Network Area [μm2] Mean” as “Variable B”. Name “Output Population” as “Syn1 density”.
18.Select “Homer1 - Number of Objects” as “Variable A” and “Network - Network Area [μm2] Mean” as “Variable B”. Name “Output Population” as “Hom1 density”.
19.Select “Homer1 - Number of Objects” as “Variable A” and “Syn1 - Number of Objects” as “Variable B”. Name “Output Population” as “Hom1/Syn1”.
20.Select “Syn1 - Number of Objects” as “Variable A” and “Syn Spots - Number of Objects” as “Variable B”. Name “Output Population” as “Syn1/Syn”.
21.Select “Hom1 - Number of Objects” as “Variable A” and “Homer Spots - Number of Objects” as “Variable B”. Name “Output Population” as “Hom1/Hom”.
22.In the Object Results building block, select “ALL” for “Homer1”, “Syn1”, and “Network” populations. Select “None” for the other population.
23.Save Columbus script via “Save Analysis to Disk”.
Analyze images of full plates
24.Open “Batch Analysis” under the Workflow menu, select the measurement to analyze (i.e., “Assay Plate” subfolder) and the analysis script in “Data Tree”, and start analysis.
25.Open “Export” under the Workflow menu and select the measurement folder (automatically generated by Columbus) in “Data Tree”. In the “Select Export Options” tab, select “Export to Disk” as “Method”, designate an “Export Folder”, select “Excel (txt)” as “Results”, and choose “Generate Subfolders” and “Include Header”.
Basic Protocol 6: SYNAPTIC DENSITY ANALYSIS
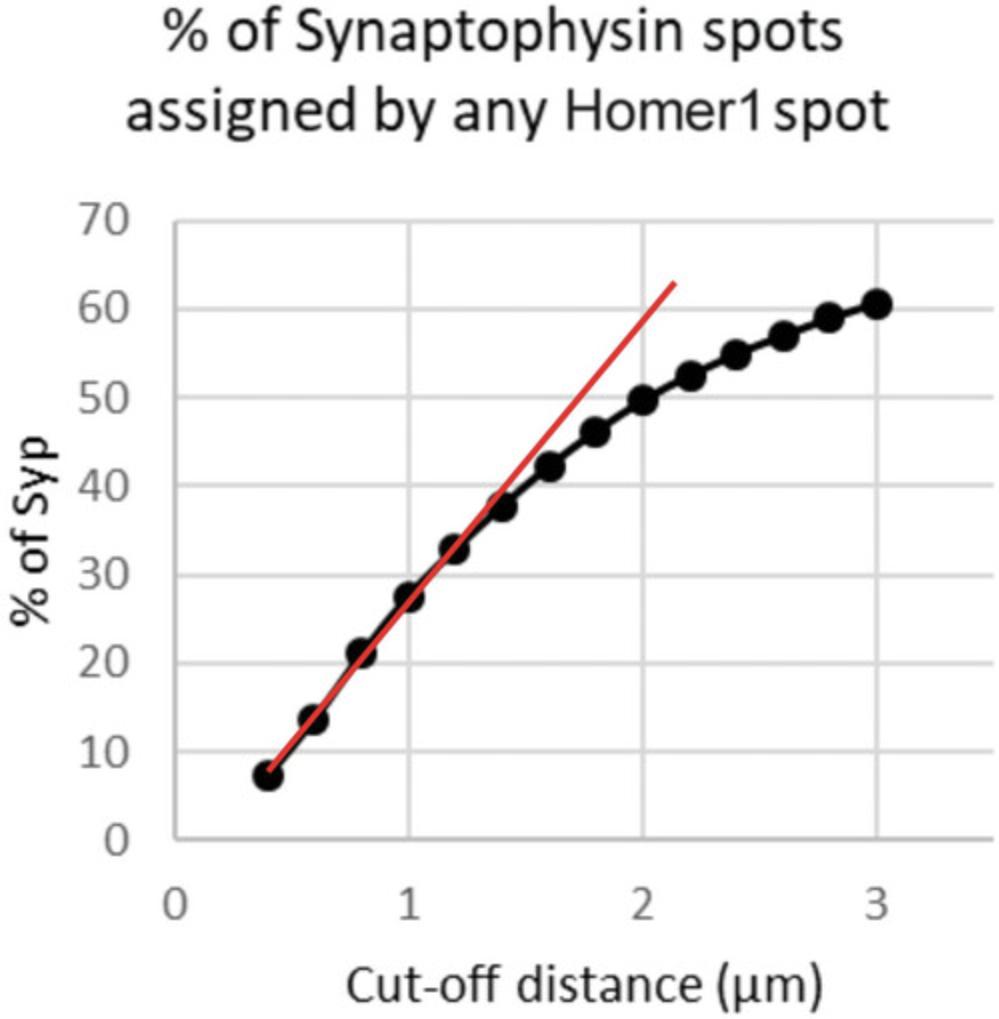
Columbus generates tabulated data that contains information for each detected synaptic spot, for each field in each well in each plate. To analyze this massive amount of tabulated data, we have generated a custom Matlab algorithm that extracts data from .txt files in stored specific folders into matrices that contain network area and network staining intensity per field, as well as xy -position and staining intensity for all Synaptophysin and Homer1 spots. The algorithm first excludes fields with no presynaptic or postsynaptic spots detected. It then identifies outlier fields within a well in terms of network area or network staining intensity. After excluding the outlier fields, the algorithm runs a subroutine to calculate, for each Homer1 spot, its Euclidian distance to all Synaptophysin spots in the same field. Each Homer1 spot is then assigned to the nearest Synaptophysin spot with a pre-determined cut-off distance. In control cultures, the fraction of Synaptophysin spots with at least one Homer1 spot assigned linearly increases with increasing cut-off distance, until it starts to reach a plateau. We thus select a cut-off distance in the linear portion of the curve and apply it to all images in the screen. The ultimate read-out of the screen, synapse density, is then defined as the area density of Synaptophysin spots with at least one Homer1 spot assigned.
Materials
- Columbus results (see Basic Protocol 5)
- Matlab, version R2017b or above (Mathworks; or similar numerical analysis software)
- Matlab code HCS_CPCB_annotated.m (see Supporting Information)
- Microsoft Excel (or similar spreadsheet software)
Copy Columbus results to a local folder and run Matlab code
1.For each plate, copy Columbus results (.txt files) into the “data” folder of the Matlab environment (or similar numerical analysis software) and run Matlab code HCS_CPCB_annotated.m.
2.Set the initial parameters in the Matlab code: “cutoffs” stands for the different cut-off distances in micrometers; “fieldnum” stands for the number of fields acquired per well; and “resolution” stands for the pixel resolution of images in µm/px.
3.Back up the three output files generated by the Matlab code (batchres.mat, display.mat, and wellnames.mat) by associating them with the screen name.
| Output parameter | Definition |
|---|---|
| 1 | Number of Syn spots (× 1000) |
| 2 | Number of Homer1 spots (× 1000) |
| 3 | % of Synaptophysin spots assigned by any Homer1 spot |
| 4 | Mean distance of assignments |
| 5 | Mean number of assignments per Synaptophysin spot |
| 6 | Mean distance of assignments (µm) |
| 7 | % of Homer1 spots assigned to any Synaptophysin spot |
| 8 | Network area (µm2) |
| 9 | Network intensity |
| 10 | Number of fields remaining after removing outliers |
| 11 | Synaptophysin1 – Intensity Spot dsRed Sum |
| 12 | Homer1 – Intensity Spot FITC Sum |
Extract Matlab results to Excel and conduct post-analysis
4.Open wellnames.mat in Matlab and copy the only column to column C of a new Microsoft Excel file.
5.Open display.mat in Matlab and copy table into columns D to CJ of the Excel file.
6.Insert three lines at the top of the sheet and label the columns as in Table 2.
| Row | Column | Well names | Number of fields (10) | Output parameter mean | Output parameter mean | Output parameter SD | Output parameter SD | ||||||||||||||||||||||||||||||||
| Cutoff 1 | Cutoff 2 | Cutoff… | Cutoff 1 | Cutoff 2 | Cutoff… | ||||||||||||||||||||||||||||||||||
| 1 | 2 | 8 | 9 | 11 | 12 | 3 | 5 | 6 | 7 | 3 | 5 | 6 | 7 | … | … | … | … | 1 | 2 | 8 | 9 | 11 | 12 | 3 | 5 | 6 | 7 | 3 | 5 | 6 | 7 | … | … | … | … | ||||
7.Complete columns A and B with the column number and the row number corresponding to each well and use these columns to sort the wells from B2 to O23.
8.Use column CK to associate each well with its respective shRNA (e.g., shNT, shSyp, shGeneX).
9.Calculate the β-score of the plate:
10.Use the data from non-transduced wells to calculate the mean of “% of Synaptophysin with no Homer assigned” for each cut-off. Plot % of Synaptophysin spots assigned by at least one Homer1 spot as a function of cut-off distance (Fig. 6).
11.Add a column to calculate “Synapse density” for each well.
12.Copy the results of all shNT control wells into a new Excel tab and calculate the means for each parameter.
13.Copy the results of all wells to a new Excel tab and normalize them using the means calculated in step 12.
14.Repeat steps 4 to 13 for each plate.
15.Copy the normalized results from each plate into a new Excel file.
16.Exclude the wells with shRNA-induced toxicity, as defined by normalized Network Area < 0.4 (<40% of control average).
17.Pool the data for the entire screen and discard shRNAs for which there are fewer than two values.
18.Identify shRNAs that have the strongest effect on synapse density.
REAGENTS AND SOLUTIONS
Borate buffer (pH 8.5), 0.1 M
- 1.55 g boric acid (Sigma-Aldrich, cat. no. B9645-500G)
- 2.375 g sodium tetraborate
- 400 ml ultrapure H2O
- Stir for 4 hr at room temperature to dissolve sodium tetraborate
- After reagents dissolve, pH should be stable at 8.5
- Bring to 500 ml with ultrapure H2O
- Filter-sterilize
- Store ≤4-6 weeks at 4°C
Culture medium
- 2 ml 50× B27 supplement (Gibco, cat. no. 17504001)
- 250 µl 100× GlutaMAX (Gibco, cat. no. 35050038)
- Bring to 100 ml with Neurobasal-A Medium (Gibco, cat. no. 10888022)
- Filter-sterilize
- Store ≤4 days at 4°C
Dissection buffer
- 2.5 ml 20× penicillin/streptomycin (Gibco, cat. no. 1507063)
- 5 ml 1 M HEPES (Gibco, cat. no. 15630056)
- 5 ml 100 mM sodium pyruvate (Gibco, cat. no. 11360039)
- 50 ml 10× Hank's Balanced Salt Solution (HBSS; Gibco, cat. no. 14185045)
- Bring to 500 ml with ultrapure H2O
- Filter-sterilize
- Store ≤6 weeks at 4°C
Dissociation medium
- 0.25 ml 20× penicillin/streptomycin (Gibco, cat. no. 15070063)
- 0.4 ml 1× MEM vitamins (Gibco, cat. no. 11120037)
- 0.5 ml 100× GlutaMAX (Gibco, cat. no. 35050038)
- 1.5 ml 20% (w/v) d-glucose (Sigma, cat. no. G8270)
- 5 ml fetal bovine serum (FBS; Gibco, cat. no. 10270106)
- Bring to 50 ml with minimum essential medium (MEM) without glutamine (Gibco, cat. no. 21090022)
- Filter-sterilize
- Store ≤4 days at 4°C
COMMENTARY
Background Information
Quantification of synaptic density can help decipher the mechanisms of synaptogenesis, synaptic plasticity, and synaptic loss observed in neurodevelopmental and neurodegenerative disorders. Here, we describe an HCS method that we applied to assess the impact of an shRNA library targeting 198 AD-associated genes on synaptic density to test the hypothesis if synapse loss driven by genetic risk factors plays a role in AD pathophysiology.
HCS combines automated imaging and quantitative data analysis in a high-throughput format suitable for large-scale applications. However, accurate assessment of synaptic density in an HCS pipeline requires mature neuronal cell cultures, adequate methods for imaging synapses, and a reliable approach to quantify synaptic density. Several HCS approaches have been developed, but most of them encountered (i) limited scalability due to the use of low-density (96-well) assay plates (Berryer et al., 2023; Jiang et al., 2020; Nieland et al., 2014), (ii) difficulties in maintaining native synaptic properties in vitro using transfection-based overexpressed synaptic proteins (Green et al., 2019), and (iii) challenges in identifying and quantifying functional/mature synapses due to the use of a single presynaptic or postsynaptic marker (Berryer et al., 2023; Spicer et al., 2017). Although several open-source methods for synaptic quantification have been developed, such as SynQuant (Wang et al., 2020), SynapseJ (Moreno Manrique et al., 2021), SynPAnal (Danielson & Lee, 2014), and ALPAQAS (Berryer et al., 2023), the HCS method we describe here is one of the few that use automated imaging of fluorescently labeled endogenous presynaptic and postsynaptic markers in mature primary neurons cultured in 384-well microplates, as well as a semi-automated image analysis workflow to quantify synapses by the proximity-based assignment of postsynaptic puncta to presynaptic puncta. Process automation ensures the throughput, accuracy, and reproducibility of our HCS, and as proof of concept, we generated data showing that our method robustly identifies modulators of synaptic density through unbiased screening of 198 shRNAs targeting AD-associated genes.
The present approach permits us not only to identify modulators of synaptic density but also to screen potential therapeutic molecules for neurodevelopmental or neurodegenerative diseases. Our future directions include the use of human induced pluripotent stem cell–derived neurons to analyze the convergent or divergent mechanisms of synaptic development and alteration between humans and rodents, as well as the use of patient-derived pluripotent stem cells for in vitro modeling of neurological diseases.
Critical Parameters
Before transducing the shRNA lentiviral library (Basic Protocol 2), it is recommended to check the quality of the PNC (Basic Protocol 1). If the cells have not adhered well to the substrate or if the neuronal network appears fragmented and/or vacuolated, it is preferable not to proceed with transduction because it is highly likely that the neurons will not survive. During the 21 days of neuronal maturation, no change of medium is needed, but it is recommended to check the level of culture medium and to add medium in the event of excessive evaporation. In our screening model, we chose to use a large number of positive and negative controls per screening plate. After optimization, the number and the semi-random distribution of control wells proved necessary to ensure the quality of our approach. As the cellular model we used is a neonatal rat neuronal culture model, it is necessary to verify that the lentiviral shRNA library used targets the rat genome. This article presents the optimal conditions for immunolabeling (Basic Protocol 3), but it is possible to adapt the protocol according to the needs of the user. Formalin use for fixation and natural donkey serum as a blocking agent are perfectly acceptable alternatives. Before starting image acquisition on a full plate (Basic Protocol 4), it is always advisable to check the quality of immunolabeling in several randomly selected wells located in different parts of the plate. Immunolabeling is highly sensitive to the ambient temperature, and image acquisition takes place at room temperature. For this reason, the reading of a plate should never exceed 8 hr of acquisition, and plates should always be stored at 4°C. Monitoring the temperature of the microscope is recommended to ensure that it does not overheat during plate reading, which would decrease the signal quality in parts of the plate. Once the image analysis has been completed, it is recommended to quickly check the quality of the acquisition using the Columbus software. To do so, one can obtain heat maps for the following parameters: Syn1 density, Hom1 density, and Network Area - Mean per Well. For Hom1 density and Network Area, values must be homogeneous within the plate (no edge or gradient effects). Syn1 density values should follow the same plate layout as the positive controls. After complete data analysis (Basic Protocols 5 and 6), any plate with a β-score below 1 should be excluded from the final results.
Troubleshooting
See Table 3 for a list of potential problems, possible causes, and suggested solutions.
| Problem | Possible cause | Solution |
|---|---|---|
| Cell death after seeding | Dissection and/or dissociation took too long | Dissection should be performed in <30 min. We recommend not to dissect more than 15 brains in one preparation. |
| Cell density is too low | Increase the cell density (≥100,000 cells/ml). Homogenize well before seeding to avoid inter-well variability. | |
| Cell death after lentiviral shRNA transduction | MOI is too high | Decrease the MOI |
| Staining shows a fragmented network | Cells dried | Never let the cells go without 1× DPBS or medium. When changing medium, always proceed column-by-column to avoid drying of the cells. |
| Over-fixed or over-permeabilized cells | Decrease the time of fixation and/or permeabilization | |
| Washing steps are too rough | Decrease the flow rate of the washer for automated aspiration/dispensation steps | |
| Weak staining intensity | Over-fixed cells | Decrease the time for fixation |
| Non-permeabilized cells | Increase the time for permeabilization | |
| Antibody concentration is too low | Use a higher concentration of antibodies or increase the time for incubation | |
| High background signal | Insufficient blocking | Increase the saturation time or consider changing the blocking agent to 5% natural donkey serum |
| Insufficient washing steps | Add one more washing step or increase the time for washes | |
| Antibody concentration is too high | Use a lower concentration of antibody | |
| Edge effect | Medium evaporates faster in the border wells than in the center wells | Check the medium level once or twice a week and add some medium if necessary. Do not seed cells in the border wells; instead, fill them with 1× DPBS. |
| Decrease in fluorescence intensity over time during acquisition | Increase in ambient temperature | The acquisition temperature must be recorded and controlled all along. Plates should always be kept at 4°C and imaged one after the other to avoid inter-plate variability. |
| Plates were left at room temperature | Always keep the plates at 4°C. Imaging should be done within <3 days. Plates should be imaged within <8 hr. | |
| β-score is <1 | Positive control is not efficient | Avoid freeze-thawing of the lentiviral shRNAs to avoid a decrease in the lentivirus titer |
| Inter-well variability is too high | Check for pipetting errors. In Basic Protocol 3, using an automatic pipet with a stepper mode (Thermo Scientific, cat. no. 46300200) can decrease the inter-well variability. | |
| Image analysis is not optimal | In the Columbus “Select Population” building block, train the computer on additional images from different wells |
Understanding Results
To reduce the risk of false negatives and false positives, we performed our screen three times using three independent neuronal cultures. Transduction of shSyp at MOI2 and MOI4 decreased the average Synaptophysin density by 69 ± 12% and 85 ± 7%, respectively. Strictly standardized mean difference (SSMD) was assessed through the β-score using positive and negative controls from each plate. Due to the inherent heterogeneity of our phenotype, a plate should be considered suitable for analysis when its β-score is >1 (Zhang et al., 2007). Among the 11 plates in our HCS study, we obtained a range of β-scores from 1.31 to 2.88 (Table 4), indicating a good level of reproducibility of our model. The synaptic density observed for each shRNA from the bank needs to be normalized by the mean synaptic density of the shNT controls from the same plate to overcome any potential inter-plate variability in terms of cell culture, immunocytochemistry, and image acquisition.
| β-score | ||
|---|---|---|
| MOI2 | MOI4 | |
| Plate 1 | 2.26 | 3.9 |
| Plate 2 | 1.66 | 1.67 |
| Plate 3 | 1.38 | 1.61 |
| Plate 4 | 2.14 | 2.67 |
| Plate 5 | 2.05 | 1.33 |
| Plate 6 | 2.82 | 2.33 |
| Plate 7 | 1.94 | 2.35 |
| Plate 8 | 1.86 | 2.67 |
| Plate 9 | 1.31 | 2.46 |
| Plate 10 | 1.33 | 1.6 |
| Plate 11 | 1.56 | 2.02 |
| Mean ± SD | 1.85 ± 0.46 | 2.24 ± 0.72 |
Time Considerations
To simplify the experimentation, we recommend preparing culture medium, coating solutions, and the intermediate dilution plate on the days prior to neuronal culture. Medium and coating solutions can be stored ≤1 week at 4°C. The first day is devoted to PNC (∼3 hr; Basic Protocol 1). Dissection should be carried out as quickly as possible (<30 min) to ensure optimum quality. The second day is devoted to transducing the lentiviral shRNA library (Basic Protocol 2). Transduction itself takes ∼2 hr, followed by a 6-hr-long incubation period before the addition of the culture medium. It then takes 21 days for the development of the neural network and the maturation of synapses. During these 21 days, the level of medium must be regularly monitored (1 to 2 times a week). Day 21 is devoted to cell fixation, saturation, and incubation with primary antibodies (Basic Protocol 3). These steps take 3 hr, including 2 hr of incubation. Day 22 is devoted to washing and incubation with secondary antibodies, followed by image acquisition. This immunostaining also takes 3 hr, including 2 hr of incubation. Image acquisition (Basic Protocol 4) must be carried out immediately after immunolabeling and usually takes 8 hr per plate. Each plate must be read immediately, one after the other. Data analysis in Columbus (Basic Protocol 5) takes a variable amount of time, depending on the training time required for successful spot detection. Approximately 10 hr is required for one screening (four plates). Data analysis in Matlab (Basic Protocol 6) takes ∼6 hr per screening plate.
Acknowledgments
The authors thank Equipex for the Equipex ImagInEx HCS platform for the robotic control and automated microscopy. This work was supported by Fondation Alzheimer (903716), Agence national de la recherché (ANR-21-CE16-0010), Sanofi iAwards Europe 2019 (196026), and Fondation pour la recherche médicale (ALZ201912009628). This work was also funded by the Lille Métropole Communauté Urbaine and the French government's LABEX DISTALZ program (Development of innovative strategies for a transdisciplinary approach to Alzheimer's disease). In addition, this work was funded by the Network of Centres of Excellence in Neurodegeneration (CoEN, pathfinder 5010).
Author Contributions
AC conducted the experiments and wrote the original draft; AC, DSW, CN, CG, AMA, FD, and EL conducted primary rat hippocampal cultures; AC, TM, and DK developed methodology and analyzed the data; AV and PB provided access to and technical help for the ImagInEx platform; JCL, DK, JD, and JC conceived the idea and managed the project. All authors reviewed and edited the manuscript.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Screening data are available from the corresponding author upon request.
Supporting Information
| Filename | Description |
|---|---|
| Aspirate.LHC10.6 KB | Program for BioTek plate washer used in Basic Protocol 3. |
| Aspirate-Dispense.LHC10.4 KB | Program for BioTek plate washer used in Basic Protocol 3. |
| HCSCPCBannotated.m14.4 KB | Program for Matlab used in Basic Protocol 6. Please note that we annotated the .m file such that readers can rewrite the code in the computational platform of their choice. |
| HCSSynapse.aas4.8 MB | Program for Columbus software used in Basic Protocol 5. |
| Washprimary.pro23.3 KB | Program for VWorks software used in Basic Protocol 3. |
| Washsecondary.pro24.2 KB | Program for VWorks software used in Basic Protocol 3. |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
Literature Cited
- Bellenguez, C., Küçükali, F., Jansen, I. E., Kleineidam, L., Moreno-Grau, S., Amin, N., Naj, A. C., Campos-Martin, R., Grenier-Boley, B., Andrade, V., Holmans, P. A., Boland, A., Damotte, V., van der Lee, S. J., Costa, M. R., Kuulasmaa, T., Yang, Q., de Rojas, I., Bis, J. C., … Lambert, J.-C. (2022). New insights into the genetic etiology of Alzheimer's disease and related dementias. Nature Genetics , 54, 412–436. https://doi.org/10.1038/s41588-022-01024-z
- Bellucci, A., Mercuri, N. B., Venneri, A., Faustini, G., Longhena, F., Pizzi, M., Missale, C., & Spano, P. (2016). Review: Parkinson's disease: From synaptic loss to connectome dysfunction. Neuropathology and Applied Neurobiology , 42, 77–94. https://doi.org/10.1111/nan.12297
- Berryer, M. H., Rizki, G., Nathanson, A., Klein, J. A., Trendafilova, D., Susco, S. G., Lam, D., Messana, A., Holton, K. M., Karhohs, K. W., Cimini, B. A., Pfaff, K., Carpenter, A. E., Rubin, L. L., & Barrett, L. E. (2023). High-content synaptic phenotyping in human cellular models reveals a role for BET proteins in synapse assembly. eLife , 12, e80168. https://doi.org/10.7554/eLife.80168
- Danielson, E., & Lee, S. H. (2014). SynPAnal: Software for rapid quantification of the density and intensity of protein puncta from fluorescence microscopy images of neurons. PLOS ONE , 9, e115298. https://doi.org/10.1371/journal.pone.0115298
- DeKosky, S. T., & Scheff, S. W. (1990). Synapse loss in frontal cortex biopsies in Alzheimer's disease: Correlation with cognitive severity. Annals of Neurology , 27, 457–464. https://doi.org/10.1002/ana.410270502
- Fogarty, M. J. (2019). Amyotrophic lateral sclerosis as a synaptopathy. Neural Regeneration Research , 14, 189–192. https://doi.org/10.4103/1673-5374.244782
- Gigg, J., McEwan, F., Smausz, R., Neill, J., & Harte, M. K. (2020). Synaptic biomarker reduction and impaired cognition in the sub-chronic PCP mouse model for schizophrenia. Journal of Psychopharmacology , 34, 115–124. https://doi.org/10.1177/0269881119874446
- Green, M. V., Pengo, T., Raybuck, J. D., Naqvi, T., McMullan, H. M., Hawkinson, J. E., Marron Fernandez de Velasco, E., Muntean, B. S., Martemyanov, K. A., Satterfield, R., Young Jr, S. M., & Thayer, S. A. (2019). Automated live-cell imaging of synapses in rat and human neuronal cultures. Frontiers in Cellular Neuroscience , 13, 467. https://doi.org/10.3389/fncel.2019.00467
- Jiang, H., Esparza, T. J., Kummer, T. T., Zhong, H., Rettig, J., & Brody, D. L. (2020). Live neuron high-content screening reveals synaptotoxic activity in alzheimer mouse model homogenates. Scientific Reports , 10, 3412. https://doi.org/10.1038/s41598-020-60118-y
- Kaech, S., & Banker, G. (2006). Culturing hippocampal neurons. Nature Protocols , 1, 2406–2415. https://doi.org/10.1038/nprot.2006.356
- Kilinc, D., Vreulx, A.-C., Mendes, T., Flaig, A., Marques-Coelho, D., Verschoore, M., Demiautte, F., Amouyel, P., Neuro-CEB Brain Bank, Eysert, F., Dourlen, P., Chapuis, J., Costa, M. R., Malmanche, N., Checler, F., & Lambert, J.-C. (2020). Pyk2 overexpression in postsynaptic neurons blocks amyloid β1-42-induced synaptotoxicity in microfluidic co-cultures. Brain Communications , 2, fcaa139. https://doi.org/10.1093/braincomms/fcaa139
- Kunkle, B. W., Grenier-Boley, B., Sims, R., Bis, J. C., Damotte, V., Naj, A. C., Boland, A., Vronskaya, M., van der Lee, S. J., Amlie-Wolf, A., Bellenguez, C., Frizatti, A., Chouraki, V., Martin, E. R., Sleegers, K., Badarinarayan, N., Jakobsdottir, J., Hamilton-Nelson, K. L., Moreno-Grau, S., Polygenic and Environmental Risk for Alzheimer's Disease Consortium (GERAD/PERADES). (2019). Genetic meta-analysis of diagnosed Alzheimer's disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nature Genetics , 51, 414–430. https://doi.org/10.1038/s41588-019-0358-2
- Mendes, T., Herledan, A., Leroux, F., Deprez, B., Lambert, J.-C., & Kilinc, D. (2020). High-content screening for protein-protein interaction modulators using proximity ligation assay in primary neurons. Current Protocols in Cell Biology , 86, e100. https://doi.org/10.1002/cpcb.100
- Monteiro, P., & Feng, G. (2017). SHANK proteins: Roles at the synapse and in autism spectrum disorder. Nature Reviews. Neuroscience , 18, 147–157. https://doi.org/10.1038/nrn.2016.183
- Moreno Manrique, J. F., Voit, P. R., Windsor, K. E., Karla, A. R., Rodriguez, S. R., & Beaudoin, G. M. J. (2021). SynapseJ: An automated, synapse identification macro for ImageJ. Frontiers in Neural Circuits , 15, 731333. https://doi.org/10.3389/fncir.2021.731333
- Nieland, T. J. F., Logan, D. J., Saulnier, J., Lam, D., Johnson, C., Root, D. E., Carpenter, A. E., & Sabatini, B. L. (2014). High content image analysis identifies novel regulators of synaptogenesis in a high-throughput RNAi screen of primary neurons. PLOS ONE , 9, e91744. https://doi.org/10.1371/journal.pone.0091744
- Spicer, T. P., Hubbs, C., Vaissiere, T., Collia, D., Rojas, C., Kilinc, M., Vick, K., Madoux, F., Baillargeon, P., Shumate, J., Martemyanov, K. A., Page, D. T., Puthanveettil, S., Hodder, P., Davis, R., Miller, C. A., Scampavia, L., & Rumbaugh, G. (2017). Improved scalability of neuron-based phenotypic screening assays for therapeutic discovery in neuropsychiatric disorders. Molecular Neuropsychiatry , 3, 141–150. https://doi.org/10.1159/000481731
- Verschuuren, M., Verstraelen, P., García-Díaz Barriga, G., Cilissen, I., Coninx, E., Verslegers, M., Larsen, P. H., Nuydens, R., & de vos, W. H. (2019). High-throughput microscopy exposes a pharmacological window in which dual leucine zipper kinase inhibition preserves neuronal network connectivity. Acta Neuropathologica Communications , 7, 93. https://doi.org/10.1186/s40478-019-0741-3
- Verstraelen, P., Garcia-Diaz Barriga, G., Verschuuren, M., Asselbergh, B., Nuydens, R., Larsen, P. H., Timmermans, J.-P., & de Vos, W. H. (2020). Systematic quantification of synapses in primary neuronal culture. iScience , 23, 101542. https://doi.org/10.1016/j.isci.2020.101542
- Wang, Y., Wang, C., Ranefall, P., Broussard, G. J., Wang, Y., Shi, G., Lyu, B., Wu, C.-T., Wang, Y., Tian, L., & Yu, G. (2020). SynQuant: An automatic tool to quantify synapses from microscopy images. Bioinformatics , 36, 1599–1606. https://doi.org/10.1093/bioinformatics/btz760
- Wong, M., & Guo, D. (2013). Dendritic spine pathology in epilepsy: Cause or consequence? Neuroscience , 251, 141–150. https://doi.org/10.1016/j.neuroscience.2012.03.048
- Zhang, X. D., Ferrer, M., Espeseth, A. S., Marine, S. D., Stec, E. M., Crackower, M. A., Holder, D. J., Heyse, J. F., & Strulovici, B. (2007). The use of strictly standardized mean difference for hit selection in primary RNA interference high-throughput screening experiments. Journal of Biomolecular Screening , 12, 497–509. https://doi.org/10.1177/1087057107300646

