FFPE Blocking and Sectioning - UMN TMCs
Laura J Niedernhofer, David A Bernlohr, Colleen Forster, HT(ASCP)QIHC
Abstract
FFPE Blocking and Sectioning protocol from BLS Histology at the University of Minnesota.
Steps
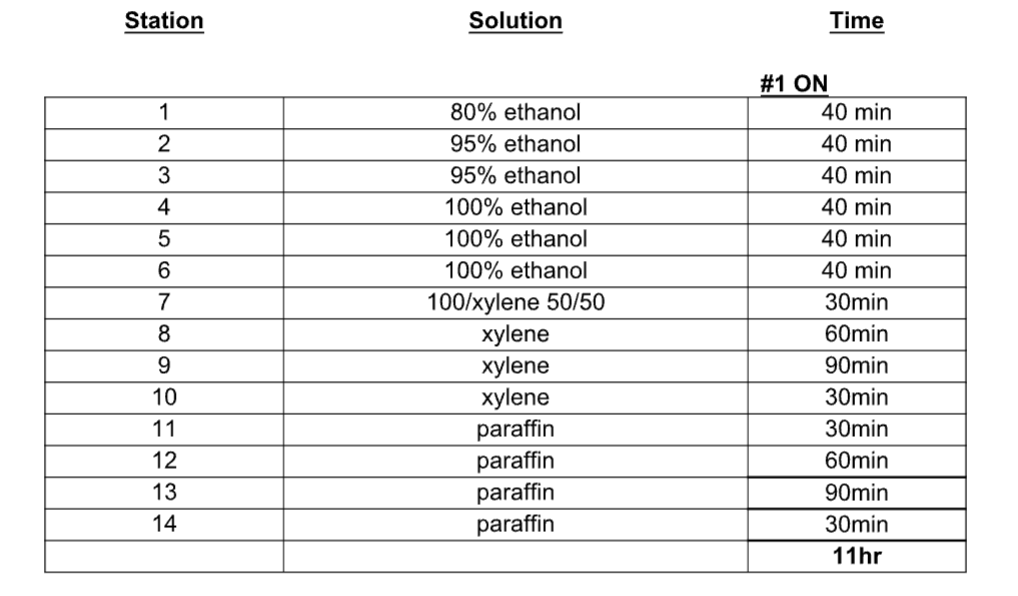
FFPE Blocking
Specimen Preparation:
The tissues for processing should be well fixed, rinsed in running tap and placed into 80%
alcohol. The suggested fixation is perfusion with 4% paraformaldehyde, fresh tissue dropped
into 4% paraformaldehyde or 10% formalin for 24-48 hours or alcohol fixed tissues.
To improve fixation, fix small pieces of tissue so solution can penetrate better.
Label cassettes using a pencil.
Block tissues according to researcher requests.
Place into labeled cassette, cover and place into container under running tap water.
Rinse well.
Drain tap water and replace with 80% alcohol
Place cassettes in paraffin bath at embedding center
Cover with paraffin.
Move to cold plate for hardening.
Pop cooled block out of mold and trim edges.
Block is ready to cut.
When cutting the tissue, prepare sections according to the Histology Request sheet.
FFPE Slide Sectioning
Remove excess wax from the sides of the plastic cassettes by scraping or melting.
Orient the block in chuck holder
Begin carefully cutting into paraffin block until a full face of tissue is exposed
Place block onto ice/water and soak for 15 minutes or longer if necessary
Cut section at desired thickness ensuring proper orientation
Carefully pick up section and place in a water bath
Place tissue section on required glass slide
Air dry for minimum of 1 hour and then move to the 37 degree oven
Dry slides overnight.