Efficient Modulation of TP53 Expression in Human Induced Pluripotent Stem Cells
Constanze Uhlmann, Constanze Uhlmann, Lisa-Maria Kuhn, Lisa-Maria Kuhn, Julia Tigges, Julia Tigges, Ellen Fritsche, Ellen Fritsche, Ulf Dietrich Kahlert, Ulf Dietrich Kahlert
Abstract
TP53 point mutations are found in 50% of all cancers and seem to play an important role in cancer pathogenesis. Thus, human induced pluripotent stem cells (hiPSCs) overexpressing mutant TP53 are a valuable tool for the generation of in vitro models of cancer stem cells or for in vivo xenograft models. Here, we describe a protocol for the alteration of gene expression in hiPSCs via overexpression of a mutant form of the TP53 (R249S) gene using lentiviral transduction. A high amount of TP53 protein is detected 1 week after transduction and antibiotic selection. Differentiation of transduced hiPSCs gives insight into better understanding cancer formation in different tissues and may be a useful tool for genetic or pharmacologic screening assays. © 2019 The Authors.
Basic Protocol 1 : Production and concentration of third-generation lentivirus
Support Protocol 1 : Cloning of gene of interest into modulation vector
Support Protocol 2 : Preparation of DMEM GlutaMAX™ with 10% fetal bovine serum and 1% penicillin-streptomycin
Basic Protocol 2 : Transduction of human induced pluripotent stem cells and selection of positively transfected cells
Support Protocol 3 : Preparation of Matrigel®-coated plates
Support Protocol 4 : Preparation of mTeSR™1 medium
INTRODUCTION
Due to their infinite availability, human induced pluripotent stem cells (hiPSCs) offer the opportunity to perform unlimited numbers of in vitro experiments. Further, hiPSC differentiation into a desired tissue allows studying physiologic processes in human-relevant cell systems. By introducing disease-specific mutations, these cells allow for studying genetic origins of disease in a tissue-specific manner with those cells. Most tumors have TP53 mutations. Among the six hot-spot mutations of TP53 , TP53 R249S (exchange of arginine for serine) is a point mutation, which causes a structural change of the protein and thereby enhances cell proliferation (Bullock & Fersht, 2001; Friedler et al., 2004; Kollareddy et al., 2015). Genetic manipulation of cells by lentiviral transduction introduces mutations in a stable and efficient manner (Naldini et al., 1996). Here, we apply this procedure to hiPSCs using a vector containing mutated TP53 (Rapti et al., 2015; Zare et al., 2016).
In this article, we report two basic protocols that have been enlisted that describe the process of hiPSC lentiviral transduction to generate genetically modified stable cell lines. In summary, Basic Protocol 1 describes the production of third-generation lentivirus with HEK 293T cells, and Basic Protocol 2 describes the generation of TP53 R249S transduced cells with the use of a lentiviral vector.
NOTE : hiPSCs are grown in standard laboratory conditions, in a humidified incubator (37°C, 5% CO2).
NOTE : Virus production and transduction of hiPSCs requires a biosafety level 2 (BSL2) laminar flow cabinet.
NOTE : All solutions and equipment must be sterile if they come in contact with cells.
Basic Protocol 1: PRODUCTION AND CONCENTRATION OF THIRD-GENERATION LENTIVIRUS
This protocol is used for the production of concentrated third-generation lentivirus. Concentrated lentivirus is produced to transduce cells (e.g., hiPSCs). All work in our laboratory is performed in BSL2 facility. However, the level of biosafety containment should be in accordance with institutional and governmental guidelines. A proper risk assessment must be done prior to beginning the work. The equipment and laminar flow hood used must be sterilized in accordance with your local rules on risk assessment when working with lentivirus.
Materials
- HEK 293T cells
- Dulbecco's modified Eagle medium (DMEM) GlutaMAX™ containing 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) penicillin-streptomycin (see Support Protocol 2)
- DMEM (e.g., Thermo Fisher Scientific, cat. no. 41965039)
- Plasmid DNA
- Third-generation lentivirus plasmids:
- pMDLg/pRRE (Addgene #12251; see Fig. 1)
- pRSV-REV (Addgene #12253; see Fig. 2)
- pMD2.G (Addgene #12259; see Fig. 3)
-
FuGENE® HD Transfection Reagent (e.g., Promega, cat. no. 231A)
-
Dulbecco's phosphate-buffered saline (DPBS) without calcium or magnesium (e.g., Thermo Fisher Scientific, cat. no. 14190094)
-
DMEM GlutaMAX™ containing 10% (v/v) FBS (e.g., Sigma-Aldrich, cat. no. F7524)
-
50% (v/v) polyethylene glycol (PEG) in distilled, deionized water
-
1.5 M NaCl, prepared in distilled, deionized water
-
10-cm plastic dishes (e.g., Greiner Bio-One, cat. no. 664160)
-
Cell culture incubator (37°C, 5% CO2)
-
0.5- and 1.5-ml microcentrifuge tube
-
15-ml conical tube
-
10-ml syringe
-
0.45-µm syringe filter (e.g., VWR, cat. no. 514-0063)
-
Vortex mixer
-
Refrigerated centrifuge (e.g., Hettich® Rotina 38R with fixed-angle rotor, cat. no.1792)
Preparation
1.Plate HEK 293T cells in a 10-cm dish with 10 ml DMEM GlutaMAX™ containing 10% FBS and 1% penicillin-streptomycin.
Day 0
2.Allow cells to reach 80% to 90% confluency. Prepare infection solution in a 1.5-ml microcentrifuge tube in the following order:
- 800 µl DMEM
- 8 µg plasmid DNA
- 4 µg pMDLg/pRRE
- 2 µg pRSV-REV
- 2 µg pMD2.G
- 60 µl FuGENE® HD.
3.Pipet solution up and down, and incubate for at least 10 min at room temperature.
4.Meanwhile, wash HEK 293T cells with DPBS.
5.Add 10 ml DMEM GlutaMAX™ containing 10% FBS without antibiotics to each dish.
6.After incubation, add infection solution dropwise to each dish. Move dish gently to distribute solution, and incubate at 37°C with 5% CO2.
Day 1
7.Remove supernatant and add 10 ml DMEM GlutaMAX™ containing 10% FBS and 1% penicillin-streptomycin.
Days 2, 3, and 4
8.To a 15-ml conical tube, add 1 ml of 50% PEG. Separately, connect syringe with a 0.45-µm filter. Pull the plunger out of the syringe out, and store it sterile in its packaging.
9.Fill syringe with supernatant, and filter supernatant into the 15-ml conical tube containing PEG by pushing the plunger.
10.Throw away syringe and filter.
11.Add 10 ml DMEM GlutaMAX™ containing 10% FBS and 1% penicillin-streptomycin to dish.
12.Add 1.2 ml of 1.5 M NaCl to each conical tube.
13.Mix solution containing supernatant, PEG, and NaCl well by vortexing or shaking, and store at 4°C until further use on day 5.
Day 5
14.Precool centrifuge to 4°C.
15.Centrifuge conical tubes (from step 13) 30 min at 7000 × g , 4°C.
16.Check if a pellet is visible.
17.After centrifugation, place tubes on ice.
18.Remove supernatant under sterile conditions, being careful not to disturb the pellet. Aspirate remaining supernatant gently with a pipette tip.
19.Resuspend pellets from all three tubes (days 2, 3, and 4) to a final volume of 400 µl DPBS (1:75 dilution of original sample volume).
20.Prepare 40-µl aliquots in 1.5-ml or 0.5-ml microcentrifuge tubes.
21.Store aliquots at −80°C or use immediately.
Support Protocol 1: CLONING OF GENE OF INTEREST INTO MODULATION VECTOR
This protocol describes the cloning procedure for inserting the TP53 R249S point mutation into a vector by a restriction enzyme–based process, leading to overexpression of the TP53 variant. The plasmid backbone is based on pSin-Ef2-Nanong-Pur (Addgene #16578; Fig. 4) and was modified with an EGFP tag at the N-terminal end, multiple cloning site, and removal of the Nanog gene sequence.
The free software SnapGene Viewer (GSL Biotech; available at https://www.snapgene.com/) was used to prepare the virtual cloning file and to design the oligomers. After cloning and purification of the plasmid from E. coli , the plasmid can be used for virus production.
Materials
-
Template for gene sequence: pLenti6/V5-p53_R249S (Addgene #22935)
-
Phusion high-fidelity DNA polymerase and buffer (e.g., New England Biolabs, cat. no. M0530L)
-
10 mM dNTP mix
-
10 µM TP53 forward oligomer: 5’-CCTTAATTAAAATGGAGGAGCCGCAGTCA-3’
-
10 µM TP53 reverse oligomer: 5’-GGAATTCCATATGTCAGTCTGAGTCAGGCCCTT-3’
-
DNA containing gene of interest (GOI)
-
Agarose
-
1× Tris-acetate-EDTA (TAE) buffer
-
SYBR® Safe DNA gel stain (e.g., Thermo Fisher Scientific, cat. no. S33102)
-
6× DNA gel loading dye (e.g., Thermo Fisher Scientific, cat. no. R0611)
-
1-kb DNA ladder (e.g., Thermo Fisher Scientific, cat. no. SM1331)
-
E.Z.N.A.® Cycle Pure Kit, V-spin (e.g., Omega Bio-Tek, cat. no. D6492)
-
10× CutSmart® Buffer (e.g., New England Biolabs, cat. no. B7204S)
-
NdeI restriction enzyme (e.g., New England Biolabs, cat. no. R0111)
-
PacI restriction enzyme (e.g., New England Biolabs, cat. no. R0547)
-
pSin-EF2 modulation vector (based on vector pSin-EF2-Nanog-Pur; Addgene #16578; see Fig. 4)
-
E.Z.N.A.® Gel Extraction Kit, V-spin (e.g., Omega Bio-Tek, cat. no. D2500)
-
10× T4 DNA ligase and buffer (e.g., New England Biolabs, cat. no. M0202)
-
TOP10 chemically competent cells (e.g., New England Biolabs, cat. no. C3019)
-
LB medium with and without 100 µg/ml ampicillin
-
10-cm LB agar plates with 100 µg/ml ampicillin (e.g., Applichem, cat. no. A0839)
-
Glycerol
-
E.Z.N.A.® Plasmid Mini Kit I, V-spin (e.g., Omega Bio-Tek, cat. no. D6943)
-
NucleoBond® Xtra Midi/Maxi (e.g., Machery-Nagel, cat. no. 740410)
-
Computer running SnapGene Viewer (available at https://www.snapgene.com/)
-
PCR reaction tubes
-
Thermal cycler
-
Spectrophotometer capable of measuring DNA concentration
-
1.5-ml microcentrifuge tube
-
16°C and 37°C incubators, with shaking capabilities
-
UV transilluminator
-
Scalpel
-
42°C heating block
-
Laboratory shaker
-
Parafilm® M (e.g., Bemis Company, cat. no. PM996)
-
15-ml conical tubes
-
Cyrovials
Preparation
Design plasmid and DNA oligomers
1.Choose a restriction site located at the multiple cloning site that cuts the vector only once. These enzymes should not cut your GOI. Use a DNA documentation program such as SnapGene Viewer to plan the cloning. To maintain the reading frame, bases might have to be added between the restriction site and the GOI. It is possible to use any base, but take care that no stop codon is generated.
2.Check which restriction enzymes cut your vector only once and not your GOI. If you have chosen two enzymes, check the efficiency of both enzymes in the restriction buffer, and if possible, use the same buffer for both enzymes.
3.Design oligomers for cloning, which include the restriction site, additional bases, and around 20 to 30 bases of your GOI. Use the table from New England Biolabs, and add bases according to the restriction enzyme (https://static.yanyin.tech/literature/current_protocol/10.1002/cpsc.102/attachments/cleavage_olignucleotides_old.pdf).
PCR amplify gene of interest
4.As a template for the GOI, use either a plasmid or cDNA.
5.In PCR reaction tubes, set up a PCR as follows (50 µl total PCR volume):
- 32.5 µl water
- 10 µl of 5× Phusion buffer
- 1 µl of 10 mM dNTP mix
- 2.5 µl of 10 µM TP53 forward oligomer
- 2.5 µl of 10 µM TP53 reverse oligomer
- 2 µl DNA
- 0.5 µl Phusion polymerase.
In the case of cDNA, plasmid DNA, and genomic DNA, dilute to 50 to 100 ng.
Use a high-fidelity polymerase with proofreading function to get a high accuracy of your GOI. This is especially important if you have a gene with a point mutation.
6.Run PCR using a thermal cycler and the following conditions:
| 1 cycle | 30 s | 98°C | (initial denaturation) |
| 29 cycles | 10 s | 98°C | (denaturation) |
| 30 s | variable | (annealing) | |
| 30 s per kb | 72°C | (extension) | |
| 1 cycle | 10 min | 72°C | (final extension) |
| Final step | indefinitely | 4°C | (hold). |
7.Prepare a 1% (w/v) agarose gel with 1× TAE and SYBR® Safe (1:10,000 dilution) to assess PCR amplification. Run 5 µl sample mixed with 6× DNA gel loading dye. Use a 1-kb DNA ladder to identify the size of your GOI. Run gel at 130 V for 15 min.
8.Purify PCR products with a positive result using the E.Z.N.A.® Cycle Pure Kit following the manufacturer's instructions.
9.Elute sample in 25 µl water. To achieve a higher concentration, prewarm water to 50°C. Measure concentration with a spectrophotometer.
10.Freeze sample at −20°C, or proceed with digestion.
Digest PCR product and vector
11.Prepare each digestion in a 1.5-ml microcentrifuge tube as follows (30 µl total volume):
- 24 µl purified PCR product
- 3 µl of 10× CutSmart® Buffer
- 1 µl restriction enzyme NdeI
- 1 µl restriction enzyme PacI
- 1 µl water.
12.Prepare digestion of modulation vector as follows (20 µl total volume):
- 2 µg undigested pSin-EF2 modulation vector
- 2 µl of 10× CutSmart® Buffer
- 1 µl restriction enzyme NdeI
- 1 µl restriction enzyme PacI
- Bring to 20 µl with water.
13.Incubate both digestions overnight at 37°C. Do not incubate longer than 15 to 16 hr.
14.Prepare a 1% (w/v) agarose gel with 1× TAE and SYBR® Safe (1:10,000 dilution). Use a comb with a 30-µl volume capacity in order to load the entire digest.
15.Mix whole digestion with 6× loading dye, and load on gel. As a control, load 200 ng undigested vector. Use a 1-kb DNA ladder as a reference for the sample size. Leave one empty well between samples and DNA ladder to prevent contamination.
16.Perform electrophoresis at 130 V for 15 min.
17.Use a UV transilluminator to visualize bands of digestion. Wear protective equipment to protect from UV light.
18.Cut out respective band with a scalpel, and place gel containing GOI in a prelabeled microcentrifuge tube.
19.Purify sample using the E.Z.N.A.® Gel Extraction Kit according to manufacturer's instructions.
20.Measure concentration.
21.Freeze sample at −20°C, or proceed with ligation.
Ligate GOI and modulation vector
22.Calculate amount of insert required for the ligation reaction using the free calculator available at http://www.insilico.uni-duesseldorf.de/Lig_Input.html. Use the following details for calculation:
- Vector size: 8324 bp
- Vector amount: 50 ng
- Insert size: 1182 bp
- Molar ratio: 1:3.
If the insert size is more than 4 kbp use a molar ratio of 1:3 and 1:5 in the software.
23.Prepare ligation as follows (10 µl total volume):
- 50 ng digested vector
- 21.3 ng digested insert
- 1 µl of 10× T4 DNA ligase buffer
- 1 µl T4 DNA ligase
- Bring to 10 µl with water.
24.Prepare ligation control as follows (10 µl total volume):
- 50 ng digested vector
- 1 µl of 10× T4 DNA ligase buffer
- 1 µl T4 DNA ligase
- Bring to 10 µl with water.
25.Incubate ligation mixture overnight at 16°C. Do not incubate longer than 16 hr.
26.The next day, proceed with transformation.
Transform
27.Let TOP10 chemically competent cells thaw on ice for 10 min. Use one vial of competent cells per ligation reaction.
28.Add 10 µl ligation mixture to chemically competent cells in a 1.5-ml microcentrifuge tube. Mix gently by tapping tube.
29.Incubate on ice for 10 min.
30.Place reaction tube in a 42°C heating block for 1 min (heat shock).
31.Stop reaction by placing cells on ice for 2 min (cold shock).
32.Add 300 µl LB medium without antibiotics.
33.Shake at 37°C for 40 min at 800 rpm.
34.Spread 150 µl cells on 10-cm LB agar plates containing ampicillin.
35.Incubate plate overnight at 37°C. Do not incubate longer than 16 hr.
36.Wrap plate with Parafilm® M, and store at 4°C until further use.
Analyze plasmid
37.Pick 3 to 5 clones from each plate, and individually add to 15-ml conical tubes containing 5 ml LB medium with ampicillin.
38.Incubate culture overnight at 37°C with shaking at 250 rpm. Do not incubate longer than 16 hr.
39.Prepare a glycerol stock of the cultures in a cryovial by adding 500 µl culture to 500 µl glycerol. Store at −80°C for further use.
40.Perform a mini-prep with the remaining culture using the E.Z.N.A.® Plasmid Mini Kit I following manufacturer's instructions.
41.Perform a digest of plasmid to control integration of the insert. As a control, digest 200 to 300 ng plasmid as described above. Stop digestion after 2 hr.
42.Check digestion on agarose gel to see whether two bands of the correct size are visible. Discard any plasmids that do not contain inserts or have fragments of the wrong size.
43.Prepare sample for sequencing according to requirements.
44.Compare sequencing results with your own vector, which contains the GOI. If it matches, proceed with the next step.
45.Prepare a midi- or maxi-prep from the positive plasmid to obtain enough material for virus production.
46.Measure concentration.
47.Freeze plasmid at −20°C until further use.
Support Protocol 2: PREPARATION OF DMEM GlutaMAX™ WITH 10% FETAL BOVINE SERUM AND 1% PENICILLIN-STREPTOMYCIN
This protocol describes the preparation of DMEM GlutaMAX**™** containing a final concentration of 10% (v/v) FBS and 1% (v/v) penicillin-streptomycin for culturing HEK 293T cells.
Materials
- FBS
- DMEM GlutaMAX™
- Penicillin-streptomycin
- 0.45-µm bottle-cap filter
1.Under sterile conditions, filter 50 ml FBS with a 0.45-µm bottle-cap filter. Add 445 ml DMEM GlutaMAX**™**.
2.Mix medium by swirling flask.
3.Remove 10 ml per 10-cm dish to be used later for virus preparation, and store at 4°C.
4.Add to the remaining medium the appropriate amount of penicillin-streptomycin. Store medium at 4°C.
Basic Protocol 2: TRANSDUCTION OF HUMAN INDUCED PLURIPOTENT STEM CELLS AND SELECTION OF POSITIVELY TRANSFECTED CELLS
This protocol can be used for transduction of hiPSCs with highly concentrated third-generation lentivirus. All work should be performed in accordance with the appropriate biosafety containment level, which is determined by institutional and governmental guidelines. The equipment and laminar flow hood used must be sterilized in accordance with local rules and risk assessment with regard to use of lentivirus.
Materials
-
hiPSC (e.g., IMR90-04; WiCell Research Institute)
-
mTeSR™1 culture medium with and without puromycin (see Support Protocol 4)
-
0.5 mM EDTA in DPBS
-
Highly concentrated lentivirus (see Basic Protocol 1)
-
Matrigel®-coated 12-well plate (see Support Protocol 3)
-
Cell culture incubator
1.Culture hiPSCs in a Matrigel®-coated 12-well plate using mTeSR™1 culture medium.
2.Split hiPSCs with 0.5 mM EDTA.
3.When cells reach 60% to 80% confluency (Fig. 5), change medium (1 ml per well), and add 1 vial (40 µl) concentrated virus to each well.
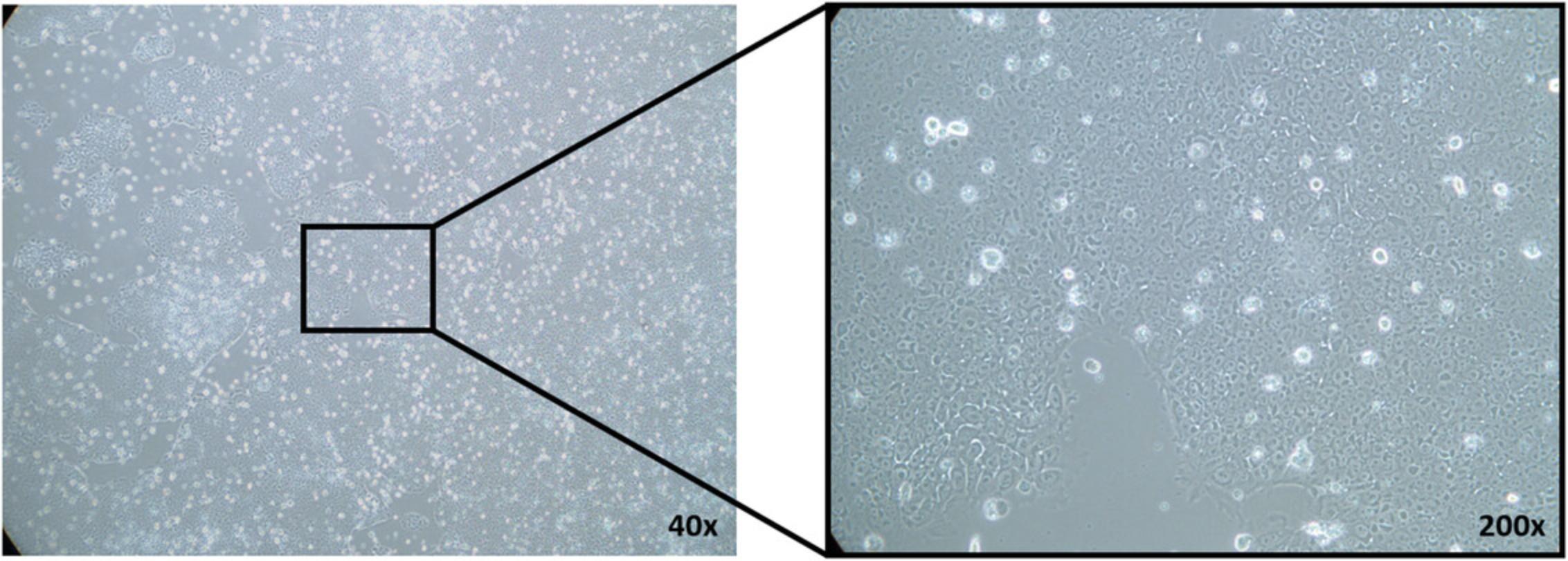
4.The next day, remove 500 µl supernatant, and add 500 µl fresh medium.
5.After 48 hr, remove all medium, and add 1 ml fresh medium.
6.Perform antibiotic selection after 72 hr using culture medium containing 1 µg/ml puromycin.
7.Split cells at least once in a plate containing medium with puromycin to achieve a more efficient selection.
8.After 1 week stop antibiotic selection, and culture cells without antibiotics.
9.Expand cell culture, and use for assays.
Support Protocol 3: PREPARATION OF MATRIGEL®-COATED PLATES
This protocol explains in short how to prepare Matrigel®-coated 6- or 12-well plates.
Materials
-
Matrigel®, hESC-qualified matrix (e.g., Corning, cat. no. 354277)
-
KnockOut DMEM (e.g., Invitrogen, cat. no. 10829018)
-
15-ml conical tubes
-
12-well plate (e.g., Greiner Bio-One, cat. no. 657160)
-
6-well plate (e.g., Greiner Bio-One, cat. no. 665180)
-
Parafilm® M (e.g., Bemis Company, cat. no. PM996)
1.Thaw Matrigel® and precool pipet tips to 4°C overnight.
2.For an even distribution, gently swirl Matrigel®, and keep on ice at all times.
3.Dilute 10 ml Matrigel® with 10 ml KnockOut DMEM. Gently pipet up and down with a precooled pipet. Prepare 0.5-ml aliquots in 15-ml conical tubes, and store at −20°C until further use.
4.To coat a plate, retrieve an aliquot from the freezer. Under sterile conditions, add an additional 1 ml KnockOut DMEM to tube, and allow to thaw.
5.Mix tube well by inverting.
6.Add 13.5 ml KnockOut DMEM, and gently mix by pipetting up and down. Prevent air bubbles and ensure everything is mixed.
7.Add 0.5 ml Matrigel®-containing solution to each well of a 12-well plate or 1 ml for a 6-well plate.
8.Swirl plate for an even distribution, and ensure the entire surface is covered. Seal plate with Parafilm® M, and incubate at room temperature for 1 hr.
9.If stored at 4°C, remove plate from refrigerator 5 min before use, and equilibrate at room temperature.
Support Protocol 4: PREPARATION OF mTeSR™1 MEDIUM
This protocol describes the preparation of mTeSR™1 medium for culturing hiPSCs.
Materials
- mTeSR™1 basal medium and 5× supplement (e.g., StemCell Technologies, cat. no. 85850)
- 10,000 U/ml penicillin-streptomycin (e.g., Pan Biotech, cat. no. P06-07100)
- 50-ml conical tubes
1.Thaw mTeSR™1 5× supplement at room temperature or at 4°C overnight.
2.On a sterile work bench, add 100 ml mTeSR™1 5× supplement to 400 ml mTeSR™1 basal medium.
3.Add 5 ml of 10,000 U/ml penicillin-streptomycin.
4.Mix well by shaking, and prepare 40-ml aliquots in 50-ml conical tubes. Store at −20°C.
5.Thaw frozen aliquots at room temperature or at 4°C overnight. Store at 4°C after thawing.
COMMENTARY
Background Information
Human cell cultures are being used to study diseases in various contexts. Frequently, patient-derived cell systems, such as cancer cell lines, are the only biological matrix used in biomedical research. Recent evidence demonstrated the limitations of cancer cell lines as a tool to use in the field of pharmacology (Ben-David et al., 2018). The low rate of success to reproduce findings of drug response when using cancer cell lines has been previously highlighted (Begley & Ellis, 2012; Haibe-Kains et al., 2013; Prinz, Schlange, & Asadullah, 2011). Recently, alternative donor-derived cancer modeling technologies, based on the introduction of defined molecular alterations in non-neoplastic cells, have emerged (Hegde, Karanikas, & Evers, 2016; Sancho-Martinez et al., 2016). Those synthetic cancer systems, as an alternative to classic cancer cell lines, may be of use in basic and translational cancer research. Here we present a detailed description of the initial steps for generation of synthetic cancer systems using hiPSCs. The use of hiPSCs is ethically accepted, and cancer models arising from a single cell of origin may be useful for fundamental basic science, as well as for translational and screening applications.
Critical Parameters and Troubleshooting
There are several critical parameters during the generation of TP53 R249S–mutated hiPSCs. Use of a highly standardized, quality-controlled hiPSC culture is of importance. The culture should be regularly checked for the expression of hiPSC markers (e.g., OCT3/4, SSEA4, Nanog, and Sox2), a normal karyotype (Steichen, Hannoun, Luce, Hauet, & Dubart-Kupperschmitt, 2019), and typical stem cell morphology with high nuclei to cytoplasm ratio and prominent nucleoli (Fig. 5; for details see Wakui et al. 2017). Successful generation of transduced hiPSCs depends further on the efficiency of the third-generation lentivirus and the ratio between cells and virus. Reducing the number of cells by reducing medium or increasing the quantity of virus by using more than one vial per experiment could improve the efficiency of the final outcome. At times, the high viral titer can be toxic to the cells; therefore, a reduction of the viral burden by dilution should be tested first (Abbasalipour et al., 2019). After transduction, select hiPSCs to obtain a homogenous culture. For antibiotic selection, a killing curve should be done to determine the optimal antibiotic dosage. Splitting/passaging cells into antibiotic-containing medium should be done on the second or third day of selection. Some promotors can be silenced over time or show no activity in stem cells (Chung et al., 2002; Xia, Zhang, Zieth, & Zhang, 2007). If cell transduction does not work, it would be best to try different promotors (e.g., EF1α; Zhang et al., 2017). At first, we used a cytomegalovirus promoter; however, after cloning the genes into an EF1α promoter–driven vector, we could transduce the hiPSCs.
Understanding Results
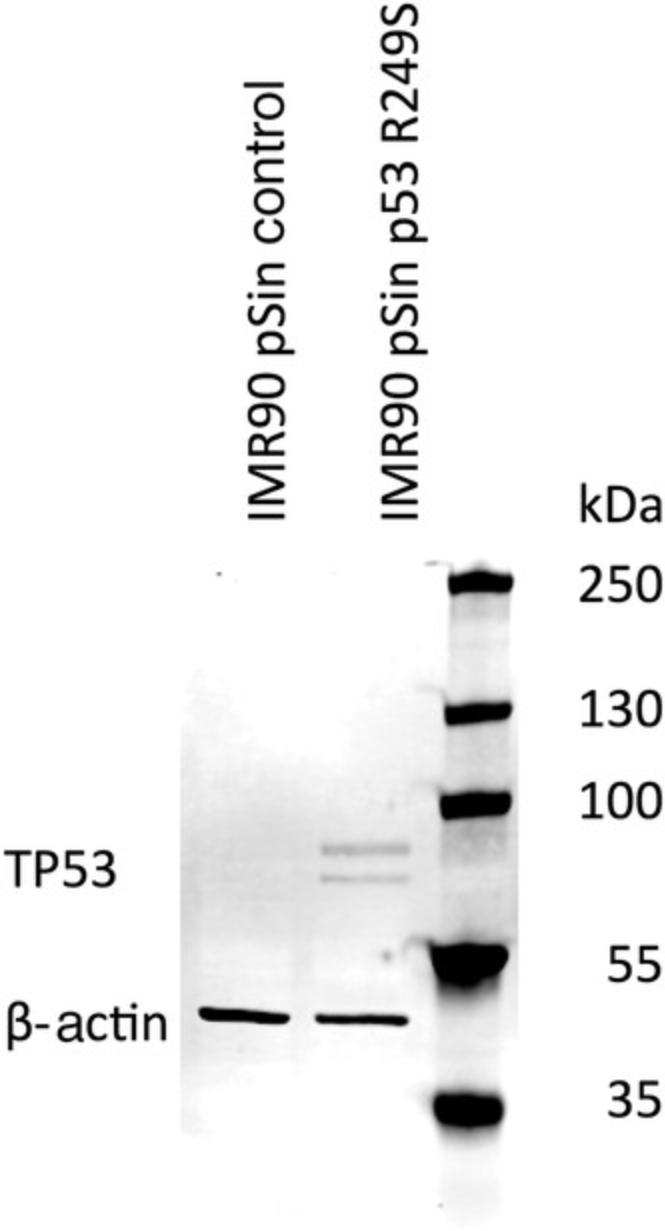
With the help of this protocol, it is possible to transduce hiPSCs with TP53 R249S. After 1 week of antibiotic selection, the cells can be expanded, and overexpression of TP53 can be detected (Fig. 6). These protocols have been replicated in another stem cell line (data not shown).
Time Considerations
Preparation of the lentivirus and transduction of hiPSCs takes about 2.5 weeks. Lentivirus production takes ∼1 week if the vector and HEK 293T cells are ready to use. After 1 week the virus is concentrated and can be used for transduction of hiPSCs. Transduction of hiPSCs requires 10 days. The virus is incubated with the hiPSCs for 3 days, and subsequently antibiotic selection begins and continues for the next 7 days. After selection, depending on the cell material, it can take up to 1 week to obtain enough cell material for the first experiments to validate expression of the molecular alteration.
Acknowledgments
This research has been funded by the Federal Ministry of Education and Research of Germany in the framework of VIP+ (BMBF, 03VP03791/92).
The authors thank Prof. Dr. Jay Gopalakrishnan and Anand Ramani (Heinrich-Heine University, Düsseldorf, Germany) for providing the modified pSin-EF2 modulation vector and cloning equipment; Anand Ramani, Gabriele Brockerhoff, and Ulrike Hübenthal for their technical support; and Ann-Christin Nickel for her help with the manuscript.
Literature Cited
- Abbasalipour, M., Khosravi, M. A., Zeinali, S., Khanahmad, H., Karimipoor, M., & Azadmanesh, K. (2019). Improvement of K562 cell line transduction by FBS mediated attachment to the cell culture plate. BioMed Research International , 2019, 9540702. doi: 10.1155/2019/9540702.
- Begley, C. G., & Ellis, L. M. (2012). Drug development: Raise standards for preclinical cancer research. Nature , 483(7391), 531–533. doi: 10.1038/483531a.
- Ben-David, U., Siranosian, B., Ha, G., Tang, H., Oren, Y., Hinohara, K., … Golub, T. R. (2018). Genetic and transcriptional evolution alters cancer cell line drug response. Nature , 560(7718), 325–330. doi: 10.1038/s41586-018-0409-3.
- Bullock, A. N., & Fersht, A. R. (2001). Rescuing the function of mutant p53. Nature Reviews Cancer , 1(1), 68–76. doi: 10.1038/35094077.
- Chen, G. (2012). Splitting hESC/hiPSC lines with EDTA in feeder free conditions. In L. Girard (Ed.), StemBook [Internet]. Cambridge, MA: Harvard Stem Cell Institute. doi: 10.3824/stembook.1.88.1.
- Chung, S., Andersson, T., Sonntag, K. C., Bjorklund, L., Isacson, O., & Kim, K. S. (2002). Analysis of different promoter systems for efficient transgene expression in mouse embryonic stem cell lines. Stem Cells , 20(2), 139–145. doi: 10.1634/stemcells.20-2-139.
- Friedler, A., DeDecker, B. S., Freund, S. M., Blair, C., Rudiger, S., & Fersht, A. R. (2004). Structural distortion of p53 by the mutation R249S and its rescue by a designed peptide: Implications for “mutant conformation”. Journal of Molecular Biology , 336(1), 187–196. doi: 10.1016/j.jmb.2003.12.005.
- Haibe-Kains, B., El-Hachem, N., Birkbak, N. J., Jin, A. C., Beck, A. H., Aerts, H. J., & Quackenbush, J. (2013). Inconsistency in large pharmacogenomic studies. Nature , 504(7480), 389–393. doi: 10.1038/nature12831.
- Hegde, P. S., Karanikas, V., & Evers, S. (2016). The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clinical Cancer Research , 22(8), 1865–1874. doi: 10.1158/1078-0432.CCR-15-1507.
- Hollstein, M., Sidransky, D., Vogelstein, B., & Harris, C. C. (1991). p53 mutations in human cancers. Science , 253(5015), 49–53. doi: 10.1126/science.1905840.
- Kollareddy, M., Dimitrova, E., Vallabhaneni, K. C., Chan, A., Le, T., Chauhan, K. M., … Martinez, L. A. (2015). Regulation of nucleotide metabolism by mutant p53 contributes to its gain-of-function activities. Nature Communications , 6, 7389–7389. doi: 10.1038/ncomms8389.
- Naldini, L., Blomer, U., Gallay, P., Ory, D., Mulligan, R., Gage, F. H., … Trono, D. (1996). In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science , 272(5259), 263–267. doi: 10.1126/science.272.5259.263.
- Prinz, F., Schlange, T., & Asadullah, K. (2011). Believe it or not: How much can we rely on published data on potential drug targets? Nature Reviews Drug Discovery , 10(9), 712. doi: 10.1038/nrd3439-c1.
- Rapti, K., Stillitano, F., Karakikes, I., Nonnenmacher, M., Weber, T., Hulot, J. S., & Hajjar, R. J. (2015). Effectiveness of gene delivery systems for pluripotent and differentiated cells. Molecular Therapy. Methods & Clinical Development, 2, 14067. doi: 10.1038/mtm.2014.67.
- Sancho-Martinez, I., Nivet, E., Xia, Y., Hishida, T., Aguirre, A., Ocampo, A., … Izpisua Belmonte, J. C. (2016). Establishment of human iPSC-based models for the study and targeting of glioma initiating cells. Nature Communications , 7, 10743. doi: 10.1038/ncomms10743.
- Steichen, C., Hannoun, Z., Luce, E., Hauet, T., & Dubart-Kupperschmitt, A. (2019). Genomic integrity of human induced pluripotent stem cells: Reprogramming, differentiation and applications. World Journal of Stem Cells , 11(10), 729–747. doi: 10.4252/wjsc.v11.i10.729.
- Wakui, T., Matsumoto, T., Matsubara, K., Kawasaki, T., Yamaguchi, H., & Akutsu, H. (2017). Method for evaluation of human induced pluripotent stem cell quality using image analysis based on the biological morphology of cells. Journal of Medical Imaging , 4(4), 044003. doi: 10.1117/1.Jmi.4.4.044003.
- Xia, X., Zhang, Y., Zieth, C. R., & Zhang, S.-C. (2007). Transgenes delivered by lentiviral vector are suppressed in human embryonic stem cells in a promoter-dependent manner. Stem Cells and Development , 16(1), 167–176. doi: 10.1089/scd.2006.0057.
- Zare, M., Soleimani, M., Mohammadian, M., Akbarzadeh, A., Havasi, P., & Zarghami, N. (2016). Efficient biotechnological approach for lentiviral transduction of induced pluripotent stem cells. Artificial Cells, Nanomedicine, and Biotechnology , 44(2), 743–748. doi: 10.3109/21691401.2014.982804.
- Zhang, M., Niibe, K., Kondo, T., Kamano, Y., Saeki, M., & Egusa, H. (2017). Gene delivery and expression systems in induced pluripotent stem cells. In K. Sasaki, O. Suzuki, N. Takahashi (Eds.), Interface oral health science 2016. Singapore: Springer.
Citing Literature
Number of times cited according to CrossRef: 6
- Pengda Qu, Shiyu Du, Wei Wang, Zhaorong Peng, Qian Hu, Haiyang Wang, Xiaohu Tang, Treatment of gouty arthritis with traditional Chinese medicine decoction: Meta-analysis, network pharmacology analysis, and molecular docking, Medicine, 10.1097/MD.0000000000036722, 103 , 1, (e36722), (2024).
- Pengda Qu, Haiyang Wang, Wei Wang, Qian Hu, Shiyu Du, Zhaorong Peng, Xiaohu Tang, Clinical efficacy evaluation and potential mechanism prediction on Guizhi-Shaoyao-Zhimu decoction in the treatment of gouty arthritis based on meta-analysis, network pharmacology analysis, and molecular docking, Medicine, 10.1097/MD.0000000000035973, 102 , 47, (e35973), (2023).
- N. Z. Mehjardi, J. Kessler, A. Y. Sanin, D. Picard, P. Westhoff, Ann-Christin Nickel, C. Uhlmann, W. Shi, H. J. Steiger, M. Remke, I. Fischer, D. Vordermark, R. S. Croner, U. D. Kahlert, The development of a hiPSC-based platform to identify tissue-dependencies of IDH1 R132H, Cell Death Discovery, 10.1038/s41420-023-01747-w, 9 , 1, (2023).
- Constanze Uhlmann, Ann‐Christin Nickel, Daniel Picard, Andrea Rossi, Guanzhang Li, Barbara Hildebrandt, Gabriele Brockerhoff, Farina Bendt, Ulrike Hübenthal, Michael Hewera, Hans‐Jakob Steiger, Dagmar Wieczorek, Aristoteles Perrakis, Wei Zhang, Marc Remke, Katharina Koch, Julia Tigges, Roland S. Croner, Ellen Fritsche, Ulf D. Kahlert, Progenitor cells derived from gene‐engineered human induced pluripotent stem cells as synthetic cancer cell alternatives for in vitro pharmacology, Biotechnology Journal, 10.1002/biot.202100693, 17 , 6, (2022).
- Dilaware Khan, Ann-Christin Nickel, Sebastian Jeising, Constanze Uhlmann, Sajjad Muhammad, Daniel Hänggi, Igor Fischer, Ulf Dietrich Kahlert, Testing the Stability of Drug Resistance on Cryopreserved, Gene-Engineered Human Induced Pluripotent Stem Cells, Pharmaceuticals, 10.3390/ph14090919, 14 , 9, (919), (2021).
- Igor Fischer, Ann-Christin Nickel, Nan Qin, Kübra Taban, David Pauck, Hans-Jakob Steiger, Marcel Kamp, Sajjad Muhammad, Daniel Hänggi, Ellen Fritsche, Marc Remke, Ulf Dietrich Kahlert, Different Calculation Strategies Are Congruent in Determining Chemotherapy Resistance of Brain Tumors In Vitro, Cells, 10.3390/cells9122689, 9 , 12, (2689), (2020).

