Completeness in reporting of surrogate primary endpoints in Randomised Controlled Trials: A targeted review protocol
Anthony Muchai Manyara, Sathish Venkatasamy Dhayalan, Philippa Davies, Amber Young, Imad Adamestam, Onyeka Obuaya, Valerie Wells, Christopher J Weir, Jane Blazeby, Rod S Taylor, Oriana Ciani
Abstract
Introduction
Using a surrogate endpoint as a substitute for a patient-relevant final outcome enables randomised controlled trials (RCTs) to be conducted more efficiently. However, there is currently no consensus-driven reporting guideline for RCTs using a surrogate endpoint as a primary outcome. Therefore, we are developing SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) and CONSORT (Consolidated Standards of Reporting Trials) extensions to improve the design and reporting of these trials. As an initial step, a targeted review will identify participants to contribute to a Delphi consensus process and document current reporting of surrogate endpoints in trial protocols and reports.
Methods and analysis
We will search for RCT reports and protocols published from 2017 to mid-2022 in six high impact general medical journals (Annals of Internal Medicine, BMJ, Journal of the American Medical Association, New England Journal of Medicine, Lancet, and PLoS Medicine) and two journals that commonly publish protocols: BMJ Open and Trials. Analysis will be done using frequencies and simple thematic analysis.
Ethics and dissemination
Ethical approval is not required. The review will support the development of SPIRIT and CONSORT extensions for reporting surrogate primary endpoints (surrogate endpoint as the primary outcome) and future evaluation of the impact of the extensions. The findings will be published in an open-access publication.
Attachments
Steps
Working definition
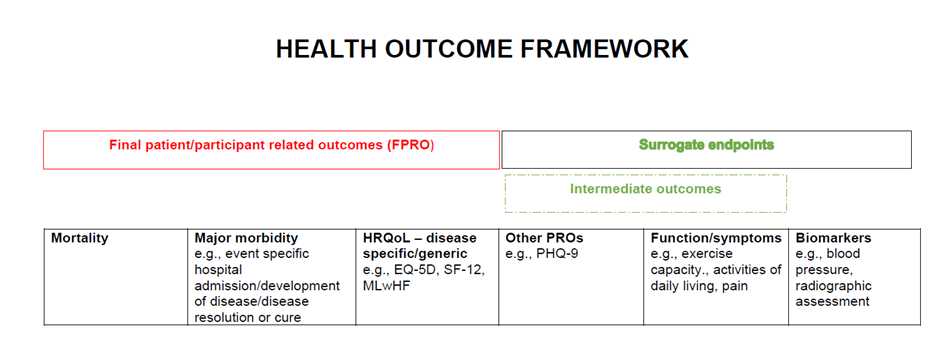
Our working definition of a surrogate endpoint is ‘A biomarker or intermediate outcome used to substitute for a patient or participant relevant final outcome (i.e., severe morbidity; health related quality of life or mortality) and reliably predicts benefit or harm based on epidemiologic; therapeutic; pathophysiologic; or other scientific evidence’. As per this definition, we will consider intermediate outcomes including symptoms and functional outcomes to be surrogate endpoints – see Figure 1 for health outcome framework below.
Search strategy and study selection
We will include RCT reports, and protocols of pragmatic and explanatory trials that use a primary outcome which is a surrogate endpoint (including if it’s part of a composite outcome) published in the last five years (2017 to mid-2022) in six high impact general medical journals ( Annals of Internal Medicine , BMJ , Journal of the American Medical Association , New England Journal of Medicine , Lancet , and PLoS Medicine) and two journals that commonly publish protocols: BMJ Open and Trials .
Journal names will be searched through PubMed and searches limited to “randomised controlled trial” and publication years (2017 to June 2022).
Identified records will be exported to Covidence [9] where title, abstract and full text screening will be done by two reviewers.
Study selection will be performed independently by two reviewers with a third reviewer providing a decision where there is lack of agreement.
Inclusion criteria
Full RCT protocols or reports including those describing follow up of completed trials.
Primary outcome is a surrogate endpoint (self-reported or considered so by reviewers) including clinical scales that measure both surrogate endpoints (e.g., symptoms and functional outcomes) and final outcomes (i.e., severe morbidity or death). Where the primary outcome is not explicitly stated, but all the outcomes are considered surrogates, such articles will also be included. Furthermore, trial protocols or reports whose primary outcome is a composite that includes a surrogate endpoint will be included. Finally, records with multiple final outcomes that include a surrogate endpoint will be included.
Exclusion criteria
We will exclude the following RCT protocols and reports:
with two primary (co-primary) outcomes that include both a surrogate and a final patient-relevant outcome, e.g., progression free survival (surrogate) and survival (final outcome).
with a primary outcome that is a clinical scale that measures both surrogate endpoints (symptoms and functional outcomes) and final outcomes (severe morbidity or death) is dichotomised to compare severe morbidity versus moderate/no morbidity than we will consider it a final outcome and exclude.
that are feasibility or pilot trials with solely feasibility objectives.
with a primary outcome that is solely safety.
that solely report cost or process evaluation analyses.
reporting a secondary analysis of a trial.
reporting non-health outcomes e.g., intelligence quotient.
Sample size and data extraction
Based on the total number of included and relevant RCTs (protocols and reports), we will calculate the proportion of these studies that are based on surrogate primary outcome.
From the included RCTs, we will select a random sample of 100 protocols and 100 reports that used a surrogate endpoint as a primary outcome for data extraction and synthesis.
Data from sample of included trials and protocols will be extracted independently by a single reviewer and checked for accuracy by a second reviewer.
We will extract study characteristics such as author, journal, year of publication, RCT hypothesis (superiority/non-inferiority/equivalence), RCT design (parallel, cross over, cluster, and number of arms), study setting (e.g., primary care, community), study country, clinical/research area, type of surrogate (i.e., biomarker or intermediate), intervention type (e.g., drug, screening), comparator (e.g., placebo, usual care), sample size, length of follow-up and funder – see https://osf.io/jr53b.
We also extract data on completeness of reporting (see below).
Assessment for completeness in reporting
We will use a list of reporting items synthesized from our recent scoping review to analyse for completeness in reporting of trials and protocols.
Items include statement of using a surrogate endpoint, rationale, and justification for using the selected surrogate, use of surrogate treatment effect or equivalent in sample size calculation, interpretation of findings in the context of limitations of surrogate endpoints among others – see full list of items in Appendix 1.
Initially we will do a pilot on the data extraction tool using six random records. Where possible and applicable, text will be extracted from papers to support coding decision.
After the pilot, synthesis for completeness will be done for all records by one reviewer and validated by a second reviewer.
Data synthesis and presentation
Given the nature of this review, synthesis of results will primarily be descriptive. Presentation of results will include PRISMA flow-chart [10] of the whole process, counts and percentages summarising study characteristics and completeness of reporting per item, and if need be, tabulation of text to support assessment of completeness judgements.

