Clusterin purification from HEK293E cells
Patricia Yuste-Checa
Abstract
This protocol details the preocedure of clusterin purification from HEK293E cells.
Attachments
Steps
Clusterin expression
Express Clusterin (Clu) in HEK293E cells cultured in FreeStyle 293 Expression Medium (Thermo Fisher Scientific, 12338018) for 96h 0m 0s.
Centrifuge culture and keep conditioned medium.
Dialyze conditioned medium in 20millimolar (mM) Na acetate 5.0 (volume ratio <1:100).
If some precipitates are observed, remove by centrifugation.
Cation exchange chromatography
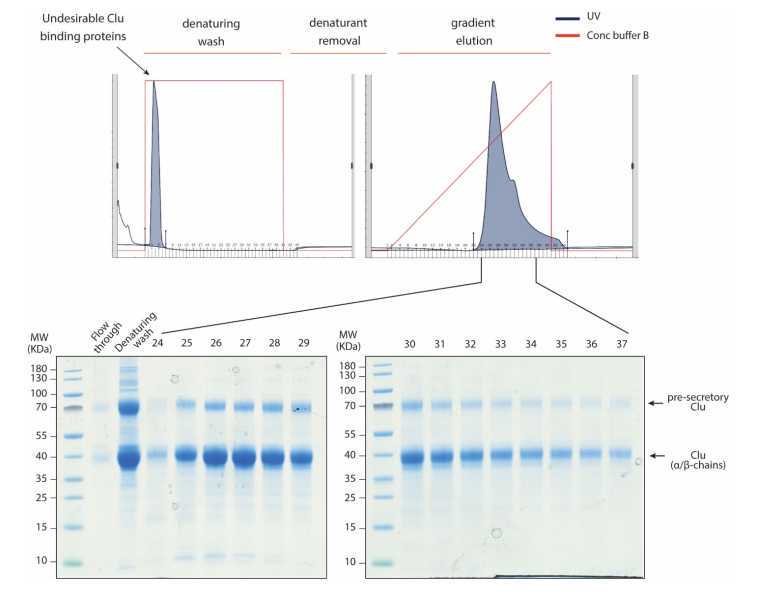
Load dialyzed conditioned medium into a HiTrap SP XL cation exchange column previously equilibrated with 20millimolar (mM) Na acetate 5.0. Wash with 20millimolar (mM) Na acetate 5.0.
Wash the column with 10 column volumes (CV) of denaturing buffer (20millimolar (mM) Na acetate 5.0, 6Molarity (M) urea) to remove undesired proteins bound to Clu.
Wash the column with 5 CVs 20millimolar (mM) Na acetate 5.0.
Elute Clu with a 0millimolar (mM)-500millimolar (mM) linear NaCl gradient in 20millimolar (mM) Na acetate 5.0.
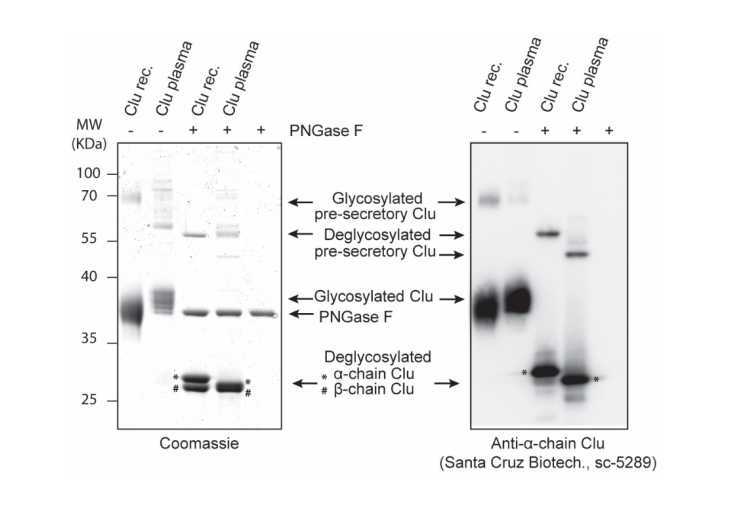
Analyze eluted fraction by SDS-PAGE and Coomassie blue staining.
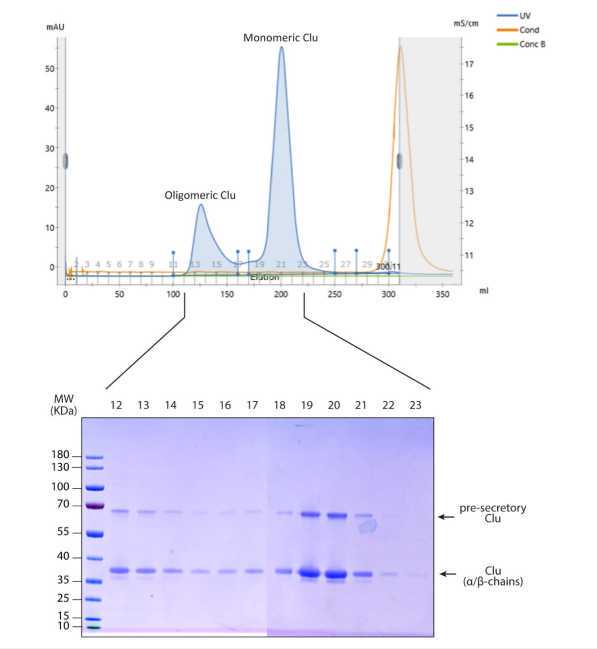
Size exclusion chromatography
Load Clu-containing fractions into a Superdex-200 previously equilibrated 20millimolar (mM) Na acetate 5.0, 100millimolar (mM) NaCl, 1millimolar (mM) EDTA.
Concentrate Clu-containing fractions using a Vivaspin ultracentrifugation unit 10,000 MWCO or similar until reach desired concentration.
Aliquot and flash-freeze purified Clu in liquid nitrogen for storage at -80°C.