Back and to the Future: From Neurotoxin-Induced to Human Parkinson's Disease Models
Mikko Airavaara, Mikko Airavaara, Ilmari Parkkinen, Ilmari Parkkinen, Julia Konovalova, Julia Konovalova, Katrina Albert, Katrina Albert, Piotr Chmielarz, Piotr Chmielarz, Andrii Domanskyi, Andrii Domanskyi
Abstract
Parkinson's disease (PD) is an age-related neurodegenerative disorder characterized by motor symptoms such as tremor, slowness of movement, rigidity, and postural instability, as well as non-motor features like sleep disturbances, loss of ability to smell, depression, constipation, and pain. Motor symptoms are caused by depletion of dopamine in the striatum due to the progressive loss of dopamine neurons in the substantia nigra pars compacta. Approximately 10% of PD cases are familial arising from genetic mutations in α-synuclein, LRRK2, DJ-1, PINK1, parkin, and several other proteins. The majority of PD cases are, however, idiopathic, i.e., having no clear etiology. PD is characterized by progressive accumulation of insoluble inclusions, known as Lewy bodies, mostly composed of α-synuclein and membrane components. The cause of PD is currently attributed to cellular proteostasis deregulation and mitochondrial dysfunction, which are likely interdependent. In addition, neuroinflammation is present in brains of PD patients, but whether it is the cause or consequence of neurodegeneration remains to be studied. Rodents do not develop PD or PD-like motor symptoms spontaneously; however, neurotoxins, genetic mutations, viral vector-mediated transgene expression and, recently, injections of misfolded α-synuclein have been successfully utilized to model certain aspects of the disease. Here, we critically review the advantages and drawbacks of rodent PD models and discuss approaches to advance pre-clinical PD research towards successful disease-modifying therapy. © 2020 The Authors.
INTRODUCTION
Disease Phenotypes
Parkinson's disease (PD) is a progressive neurodegenerative disease lacking disease-modifying therapies, and current drug treatments are symptomatic. The motor symptoms of PD are rigidity, tremor, postural instability, and bradykinesia, but also non-motor signs such as lack of motivation, depression, sleep disorders, loss of smell, cognitive impairment, and constipation are present (Bartels & Leenders, 2009; Halliday, Lees, & Stern, 2011; Weintraub & Burn, 2011). PD is characterized by slow and progressive age-related degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc) projecting to the dorsal striatum, which are part of the circuitry controlling motor learning and movement (Moore, West, Dawson, & Dawson, 2005).
Why do dopaminergic neurons in the SNpc start to die? We do not know the exact answer to this question. Considering that most PD cases are idiopathic it is likely that there is a deregulation of basic cellular functions that cause the disease. The loss of dopaminergic neurons in familial forms of PD correlates with mutations in genes such as SNCA, Parkin/PARK2, ubiquitin carboxy terminal hydrolase-1 (UCHL1), PINK1, DJ-1/PARK7, and LRRK2 (Gasser, Hardy, & Mizuno, 2011; Nalls et al., 2014). In addition, several cellular and molecular mechanisms such as mitochondrial dysfunction, proteostasis, lysosomal and autophagy failures, oxidative stress, and neuroinflammation may underlie the disease (Hirsch & Hunot, 2009; Obeso et al., 2010; Thomas & Beal, 2007; Valente, Arena, Torosantucci, & Gelmetti, 2012). Since there is no single cause for the neurodegeneration, one option is that selective vulnerability of SNpc neurons–over other dopaminergic neurocircuitry–is caused by their physiological properties. For example, L-type Ca2+ and K+ ATP channels mediating the pacemaking activity specific for SNpc dopaminergic neurons can cause increased stress (Chan et al., 2007; Guzman et al., 2010; Liss et al., 2005; Mosharov et al., 2009; Nedergaard, Flatman, & Engberg, 1993). In addition, extensive arborization of nigrostriatal dopaminergic neurons as compared to other neurons (Matsuda et al., 2009) increases their demand for energy, proteostasis, and lysosomal functions.
The presence of α-synuclein-containing insoluble inclusions in neuronal soma or neurites, called Lewy bodies or Lewy neurites, respectively, is the major histopathological hallmark of PD (Spillantini, Crowther, Jakes, Hasegawa, & Goedert, 1998). Under normal conditions, α-synuclein, an abundant protein located in presynaptic terminals, exists in monomeric, oligomeric, and aggregated forms in equilibrium (Bengoa-Vergniory, Roberts, Wade-Martins, & Alegre-Abarrategui, 2017). However, under unknown pathological conditions, Lewy bodies and Lewy neurites are formed and spread spatially and temporally in a prion-like fashion (Braak et al., 2003; Kordower, Chu, Hauser, Freeman, & Olanow, 2008; Li et al., 2008). Although α-synuclein inclusions in sporadic PD as well as mutations in the SNCA gene coding this protein point out its relation to the disease pathology, mechanisms linking Lewy body formation and neurodegeneration are still poorly understood. There are several pre-clinical studies that suggest the toxicity of α-synuclein inclusions causes degeneration of dopaminergic neurons. In 2003, Heiko Braak and colleagues proposed a classification (Braak stages) of the degree of progressive PD pathology based on the presence of Lewy bodies (Braak et al., 2003). However, a review by Robert Burke and colleagues already in 2008 noticed that there is no correlation between Braak stages and clinical severity of PD (Burke, Dauer, & Vonsattel, 2008). Moreover, a post-mortem study comparing Lewy bodies and dopaminergic neuron density in the SNpc showed that neuronal density was not related to the Braak PD stage or Lewy body densities in the cortex (Parkkinen et al., 2011). In addition, the age at death or duration of disease did not have any effect on the proportion of nigral dopaminergic neurons with Lewy bodies (Parkkinen et al., 2011). Thus, it is rather likely that Lewy bodies are not the toxic component responsible for cell death. However, Lewy bodies can cause functional deficits in neurons, which may lead to many of the symptoms of PD, although this needs to be studied further.
Dopaminergic Neurodegeneration—Losing the Phenotype Versus Cell Death
We have previously reviewed the concept of neurorestorative therapies (Domanskyi et al., 2015). It is based on clinical and animal studies revealing that the “dying back” degeneration of nigrostriatal dopaminergic neurons starts from the caudate putamen (also known as striatum) and progresses to cell bodies in the SNpc (Bernheimer, Birkmayer, Hornykiewicz, Jellinger, & Seitelberger, 1973; Kish, Shannak, & Hornykiewicz, 1988; Scherman et al., 1989). In dying nigrostriatal dopaminergic neurons labeled with green fluorescent protein (GFP), GFP fluorescence was still detected after the phenotypic markers were lost (Cheng et al., 2011). In addition, axotomized cholinergic neurons first lose their phenotypic markers, but retain cytoarchitecture, before they die (Hagg, Manthorpe, Vahlsing, & Varon, 1988; Williams, 1996). A similar phenomenon occurs in toxin models (Airavaara, Voutilainen, Wang, & Hoffer, 2012; Schober, 2004) and in genetic rodent models of PD (Domanskyi, Alter, Vogt, Gass, & Vinnikov, 2014; Ekstrand et al., 2007; Y. Li et al., 2009; Rieker et al., 2011). Moreover, the estimations carried out by Richard Burke and colleagues show that at the onset of PD symptoms only approximately 30% of SNpc dopaminergic neurons are lost (Cheng, Ulane, & Burke, 2010), indicating that the majority of dopaminergic neurons are viable at this time point. This loss of phenotype phenomenon before actual neuronal death gives a possibility to apply therapies aiming to restore the dopaminergic phenotype and function as long as neuronal cell bodies are still present. This concept is nevertheless different from regeneration therapies where new neurons are induced either from endogenous or transplanted neuronal precursors aiming at repairing and replacing the degenerating dopamine cells or circuitry.
In neurorestoration, there are dopaminergic neurons that do not have functional synaptic contacts and axons have lost their phenotype in a gradient manner progressing from the striatum. These neurons can be targets for neurorestorative disease-modifying therapy. The longer and faster neurodegeneration progresses, the smaller the number of neurons will be that can be restored. However, when the neurodegeneration has progressed too far there may be no more possibility to restore these cells. Naturally, PD progresses at different rates and we need more quantitative measures to follow the progression of neurodegeneration in PD patients.
How to Measure Neuroprotection and Neurorestoration of SNpc Dopaminergic Neurons?
Neuroprotective and neurorestorative effects need to be shown in multiple ways. In order to conclude that a given treatment is protective, one needs to quantify dopaminergic neurite and cell soma restoration, and protection of functionality needs to be shown with behavioral analysis. The advantage of animal PD models is that because the nigrostriatal dopamine system has such a strong role in controlling behavior, neurodegeneration can be estimated by measuring behavioral deficits. The most used behavioral tests for a unilateral lesion are cylinder test, D-amphetamine-induced rotations, and the staircase test (Asakawa et al., 2016; Montoya, Campbell-Hope, Pemberton, & Dunnett, 1991). Behavioral functional recovery needs to correlate with neurite density or dopamine concentrations in the striatum. Neurite density can be measured with markers such as tyrosine hydroxylase (TH), VMAT, or dopamine transporter (DAT), with TH being the most sensitive for up or downregulation with various stimuli (Kumer & Vrana, 1996; Tenenbaum & Humbert-Claude, 2017). Behavioral effects do not need to directly correlate with dopaminergic neuron numbers in SNpc, but naturally the neuroprotection needs to be shown at the neuron number level as well.
Conversely, several studies have shown the loss of neurites or cell bodies in rodent PD models, but without having behavioral deficits the data does not make much sense. If the dopaminergic neurons are depleted and the neurites gone, the mobility of animals should be minimal. Indeed, the animal should have severely reduced mobility, similar to Arvid Carslsson's rabbits after reserpine-induced parkinsonism, where reserpine depletes amine stores (Carlsson, 2001; Carlsson, Lindqvist, & Magnusson, 1957). However, one cannot claim a neuroprotective effect with behavioral improvement alone unless it is paired with a cellular and molecular outcome. There can be many reasons for the treatment to cause behavioral improvement after dopaminergic degeneration, other than direct effects on the nigrostriatal dopamine system. Thus, while behavioral responses after a unilateral or bilateral lesion can be measured with established tests, without quantification of dopaminergic neurite density and neuron numbers, these tests are not enough to draw conclusions on neuroprotection and neurorestoration after a given treatment. There is much that we can do as a field to improve the quality of our studies in this regard.
NEUROTOXIN-INDUCED MODELS
6-Hydroxydopamine (6-OHDA)
Dopamine is synthesized enzymatically, by TH and AADC, from the amino acids L-tyrosine and L-phenylalanine (Meiser, Weindl, & Hiller, 2013; Senoh & Witkop, 1959). First, L-phenylalanine is oxidized into L-tyrosine by phenylalanine hydroxylase, which is oxidized into DOPA by TH and finally DOPA is decarboxylated into dopamine by AADC. The alternative synthesis of dopamine happens by AADC decarboxylating L-phenylalanine into phenylethylamine which is oxidized into tyramine, or L-tyrosine into tyramine. Tyramine is then oxidized into dopamine by CYP2D. Dopamine is further oxidized into noradrenaline and both dopamine and DOPA can be oxidized enzymatically into toxic metabolites, by catalysis (Fe3+) or by reactive oxygen species (ROS) to the highly reactive ortho-quinones DOPA-quinone and dopamine-quinone. Dopamine-quinones are precursors to many neurotoxins that mainly cause oxidative stress and mitochondrial dysfunction by blocking the function of the electron transport chain. If iron is present, dopamine-quinone can react to form the neurotoxin 6-hydroxydopamine (6-OHDA) (Graham, 1978; Napolitano, Pezzella, & Prota, 1999) (Fig. 1).
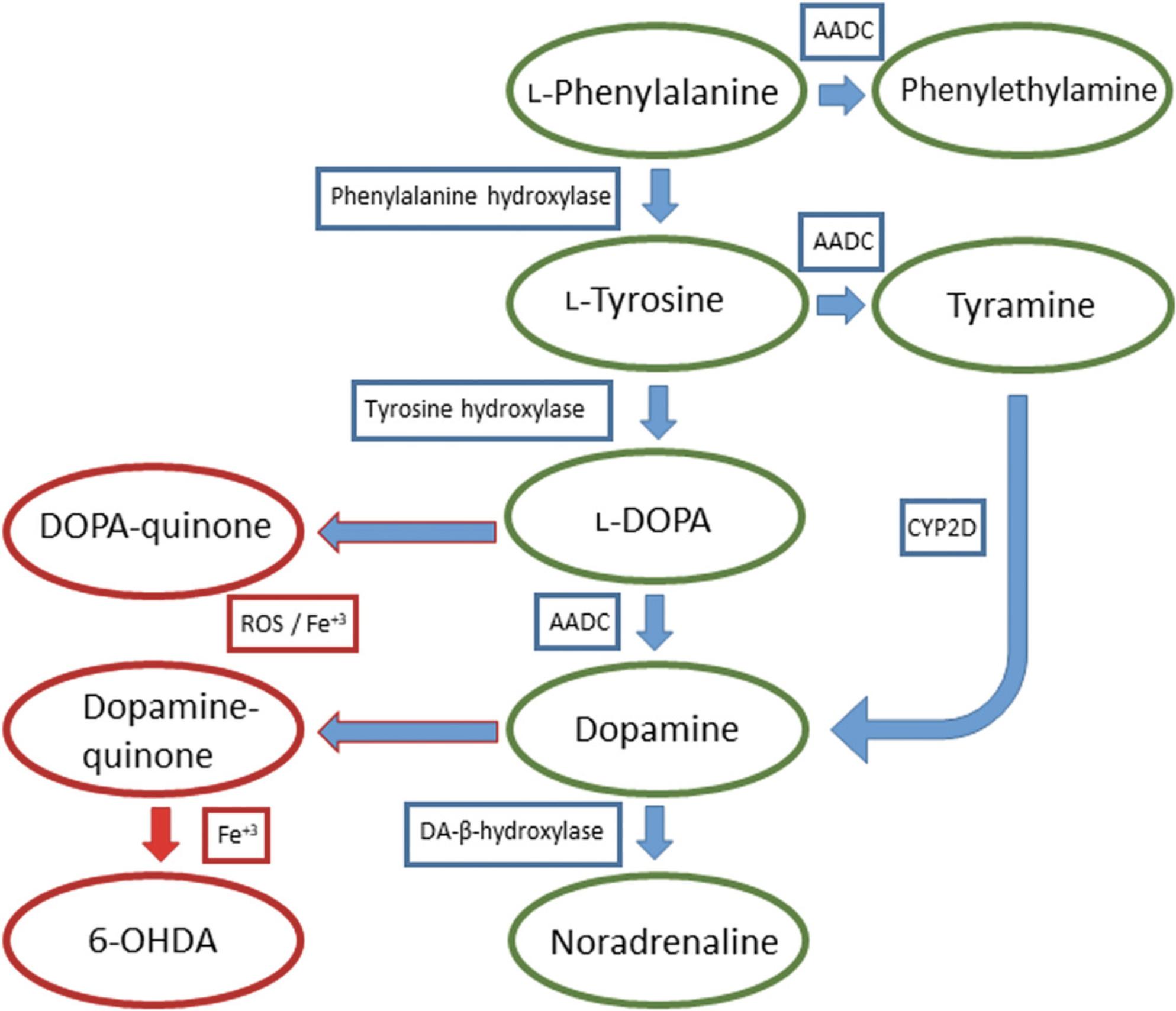
6-OHDA internalizes to dopaminergic and noradrenergic neurons through the DAT and noradrenaline transporters (NET), respectively, and its cytotoxic effects are thought to be mediated mainly by oxidative stress, caused by the generation of ROS by either blocking the mitochondrial respiratory chain complex I or formation of hydrogen peroxide and various radicals, but the exact mechanisms are still unclear (Glinka, Gassen, & Youdim, 1997; Luthman, Fredriksson, Sundstrom, Jonsson, & Archer, 1989; Mazzio, Reams, & Soliman, 2004; Saner & Thoenen, 1971). Although it is selectively taken up by these neurons, it can cause some damage to other neurons non-specifically. A plethora of other effects have been associated with 6-OHDA, for example, neuroinflammation and activation of microglia, which may contribute to the degeneration of dopaminergic neurons (Cicchetti et al., 2002). It should also be noted that in many protocols 6-OHDA is diluted in sterile saline containing ascorbic acid, and thus, acidic solution itself can cause activation of glial cells.
6-OHDA was discovered to cause degeneration of the nigrostriatal system in the late 1960s (Ungerstedt, 1968). In the 1970s, it was used to develop the first animal model of PD (Ungerstedt, 1971b; Ungerstedt & Arbuthnott, 1970; Ungerstedt, Ljungberg, & Steg, 1974). Many in vivo models using 6-OHDA have been established as it works well in various animals, mainly rodents (mice, rats, and guinea pigs), cats, and primates. There are three main sites in the nigrostriatal pathway where 6-OHDA can be injected for modeling PD: the medial forebrain bundle (MFB), SNpc, and striatum (Faull & Laverty, 1969; Hefti, Melamed, & Wurtman, 1980; Kirik, Rosenblad, & Bjorklund, 1998; Perese, Ulman, Viola, Ewing, & Bankiewicz, 1989; Sauer & Oertel, 1994; Stanic, Finkelstein, Bourke, Drago, & Horne, 2003). Since dopaminergic cell bodies are located in the SNpc and their projecting axonal terminals in the striatum, the most prominent lesion and rapid cell death (within days) comes when injecting 6-OHDA to the nigra/MFB, while the mildest lesion and slower cell death (weeks) is observed when injecting to the striatum. Unilateral lesions are preferred as bilateral lesions have a significantly higher mortality rate due to increased aphagia, adipsia, and seizures (Sakai & Gash, 1994; Ungerstedt, 1971a). Studies carried out in the MFB show that apomorphine-induced rotations are a good estimate when predicting the lesion success, and more precise than amphetamine-induced rotations (Hudson et al., 1993). Follow-up studies have shown that apomorphine at a dose of 0.05 mg/kg works well in Fisher rats as described earlier (Hudson et al., 1993), but in Han Wistar rats we were not able to see similar effects with such a low dose (Huotarinen et al., 2018). Strain differences are common and we would recommend this model to be adopted based on the Hudson et al. (1993). Another issue with apomorphine-induced rotations in MFB lesioned animals is rapid development of sensitization, and already a second injection of apomorphine causes a robust but rather short conditioning effect (Hudson, Fong, Boyson, & Hoffer, 1994). This issue can be avoided by habituating the animals before the injection of apomorphine.
One of the first thorough characterization and optimization studies on the striatal unilateral lesion were done in the 1990s (Kirik et al., 1998; Przedborski et al., 1995). They observed that the lesion depends mostly on the injection site(s) and dosage. These parameters have since been further optimized by others for further stability (Heuer, Smith, Lelos, Lane, & Dunnett, 2012; Penttinen et al., 2016). Comparing various dosing regimens, needles, and injection sites, we have found that the most reliable partial lesion and behavioral effect, analyzed by TH staining and drug-induced rotations, was obtained by injecting 3 × 2 µg of 6-OHDA into three regions of the striatum of rats (Penttinen et al., 2016). This low-dose model produces consistent lesions, and therefore, reduces the number of animals needed for experiments.
The disadvantage of the unilateral striatal 6-OHDA model is that it does not replicate human PD very well as the loss of dopaminergic neurons progresses fast (days-weeks) compared to patients (years) and there is a lack of hallmarks, such as Lewy bodies or other filamental intraneuronal inclusions (Muma et al., 2001; Zeng et al., 2002). 6-OHDA does not cross the blood-brain barrier, so it needs to be administered intracranially (intraventricularly, intracisternally, or intracerebrally) by stereotaxic injections, and the rat is preferred as the injection sites are quite small, and thus, requires more precision in mice (Ungerstedt, 1968, 1971b). A norepinephrine transporter (NET) inhibitor, such as desipramine, needs to be administered concomitantly to achieve dopaminergic selectivity. 6-OHDA is also quite unstable and light sensitive, so it should be prepared immediately before the experiment and protected from light at all times (Soto-Otero, Mendez-Alvarez, Hermida-Ameijeiras, Munoz-Patino, & Labandeira-Garcia, 2000). The liquid changes color from clear to dark brown when 6-OHDA is oxidized, so the stability can be confirmed by visual inspection.
The advantages of the unilateral striatal 6-OHDA model, however, are that it replicates the degeneration of the nigrostriatal tract and this effect is quite reproducible and well-tolerated by the animals (Penttinen et al., 2016). The effects on locomotion can also be quantified reliably so it is very useful in screening for new therapeutics aimed at restoring or protecting the mesencephalic dopamine system (Penttinen, Parkkinen, Voutilainen, et al., 2018; Voutilainen et al., 2017). Behavioral assessment is much easier with regards to locomotor activity and the use of the contralateral side as a control enables reliable and physiologically relevant comparison in biochemical assessment of the lesion (Ungerstedt & Arbuthnott, 1970). The unilateral 6-OHDA model is also widely used in modeling dyskinesias in conjunction with chronic L-DOPA treatment (Lundblad et al., 2005; Smith, Heuer, Dunnett, & Lane, 2012). In addition, since it is a unilateral model, there are very few ethical concerns because the animals’ feeding and drinking behavior is not altered.
Behavioral assessment is done mostly by drug-induced asymmetrical rotations, mainly dopamine receptor agonists or dopamine releasing compounds such as D-amphetamine and apomorphine, or alternatively by the cylinder test (where no drugs are used) (Hefti et al., 1980; Schallert, Fleming, Leasure, Tillerson, & Bland, 2000; Ungerstedt & Arbuthnott, 1970). In rotations, after unilateral 6-OHDA model induction, the animal is injected with a relatively high dose of D-amphetamine to induce ipsilateral rotations or apomorphine to induce contralateral rotations, which are quantified for a certain amount of time, usually 2 hr at most, and measure the locomotor deficits caused by the loss of dopamine in the striatum. The rotation test has received criticism due to the usage of pharmacological agents that may interfere and/or affect the outcome of the studied interfering factor, especially when they are pharmacological interventions (Marin, Rodriguez-Oroz, & Obeso, 2006). Animals may sensitize to amphetamines and correlation with nigral cell loss is not very high. Thus, in many cases the cylinder test can be used as a non-pharmacological test or the limb-use asymmetry test (Schallert et al., 2000). It measures activity/novelty seeking behavior and locomotion by quantifying the amount and difference of ipsi- and contralateral paw touches, rearings, and paw touches after rearings for a set amount of time, usually between 5 and 20 min, in a cylindric apparatus. Other motor performance tests such as stepping test or adhesive removal test can also be implemented. In addition, non-motor symptoms in the 6-OHDA models have been assessed with various behavioral tests, some of them producing robust effects, but not to the same extent as the motor phenotype tests (Bonito-Oliva, Masini, & Fisone, 2014).
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)
Loss of dopaminergic circuitry can be induced in C57/Bl6 mice by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) i.p. injections (Heikkila, Nicklas, Vyas, & Duvoisin, 1985; Jonsson et al., 1985; Sundstrom, Stromberg, Tsutsumi, Olson, & Jonsson, 1987). Since these studies, MPTP has been used extensively for almost 30 years. MPTP produces a PD-like syndrome in non-human primates and has been shown to cause parkinsonism in humans. Although this toxin does not reflect all proximal causes of PD or its neuropathology, and clinical condition of PD occurs progressively over many years, there are several reasons to use this model. First, MPTP appears to produce key aspects of the cellular effects believed to be involved in the motor symptoms of PD and to produce a robust phenotype both in terms of the loss of dopaminergic neurons and severe motor deficits. Second, MPTP can be used in mice, monkeys, and in tissue culture via its active metabolite, MPP+. In mice, the MPTP model can be divided into three categories: acute, subacute, and progressive.
In the acute model, MPTP is given four times at 2-hr intervals at doses between 15 and 20 mg/kg (Airavaara, Harvey, et al., 2012; Chen et al., 2008; Jackson-Lewis & Przedborski, 2007). Our experience in the model is that the PD motor phenotype can be variable and this variation may result from the stress status of the animal. For example, if the male mice have been fighting the outcome of MPTP lesion can vary. The second thing that we have observed is that the lethality can be high and this can be avoided by simply putting the mice into cages without bedding and keeping them there for a day after injections. In the subacute model, mice receive five consecutive doses of MPTP 25 mg/kg i.p. (Ghosh et al., 2016). The subacute model causes less acute lethality and the loss of dopaminergic phenotype is more consistent. The third is a “progressive model” of MPTP administration in which MPTP is given every 3.5 days over 5 weeks, each injection preceded by probenecid to increase the retention of MPTP in the CNS (Petroske, Meredith, Callen, Totterdell, & Lau, 2001). This model leads to the gradual onset of behavioral and dopaminergic impairment, allowing an experimenter to introduce intervention during the earliest neurodegeneration stage. Also, unlike the acute MPTP model, these mice develop α-synuclein-positive inclusions and changes in the folding of proteins (Fornai et al., 2005). There seems to be some spontaneous recovery in all of these MPTP models, but the MPTP/probenecid model causes the most long-lasting deficits, and even after 6 months post MPTP/probenecid almost 80% of striatal dopamine is still depleted.
The disadvantage of MPTP is that due to the bilateral lesion it also causes PD-like symptoms in general mouse behavior. Therefore, one needs to carefully take care of nutrition and hydration status of the animals. The other disadvantage where careful consideration needs to be taken is that this model works well only in C57Bl6 mice. In other mouse strains the effects are more variable, and it does not work in rats at all. One should also pay attention in selecting the C57Bl6 mouse sub-strain, since some of them are knockouts for α-synuclein (Specht & Schoepfer, 2001). MPTP can also be used in combination with genetic modifications; however, due to differential sensitivity to MPTP one should be careful when using mice from mixed genetic backgrounds.
Lactacystin
Protein metabolism has been a central interest in PD due to observed lysosomal dysfunction, especially after the discovery that Lewy bodies contain misfolded proteins, such as α-synuclein, and membrane fragments (Shahmoradian et al., 2019; Spillantini et al., 1998). Further discoveries of genes implicated in familial PD and involved in the ubiquitin-proteasome system, namely UCHL1 and parkin, raised awareness on the importance of protein metabolism (Kitada et al., 1998; Leroy et al., 1998). These new insights were followed by other findings in proteasomal dysfunction which led to the development of new animal models of PD (McNaught & Jenner, 2001; McNaught et al., 2002; McNaught, Belizaire, Jenner, Olanow, & Isacson, 2002). Proteasomal inhibitors, namely lactacystin and PSI, were found to selectively kill dopaminergic neurons in the nigrostriatal system, reduce dopamine levels in the striatum, and cause abnormalities in motor function. Lactacystin has been tested in various animal models, notably fish, rodents, and primates and at different administration sites, mainly, as with 6-OHDA, the SNpc, MFB, and striatum, as well as systemically (Bentea, Verbruggen, & Massie, 2017). Loss of dopaminergic neurons has been reported with impaired motor behavior and filamental proteinaceous inclusions in brain regions affected by PD. However, later studies could not reproduce most of these findings, although some aspects of the model, such as the loss of nigral dopaminergic neurons and accumulation of proteinaceous inclusions, have been replicated (Savolainen, Albert, Airavaara, & Myohanen, 2017). In addition, the observed motor deficits were not as prominent as in the original studies (Kordower et al., 2006; Manning-Bog et al., 2006). More recent studies have optimized lactacystin dosage or used it in combination with other agents such as LPS to observe more robust responses (Deneyer, Albertini, Bentea, & Massie, 2019).
Pesticides
Rotenone, reported to be used as a fish poison in Peru in 1929, is a pesticidic flavonoid found in many Leguminosa plants and causes selective nigrostriatal dopaminergic degeneration by inhibiting mitochondria complex I and proteasomal activity, causing oxidative and proteolytic stress (Betarbet et al., 2000; Clark, 1929; Sherer et al., 2003). It causes accumulation of Lewy body-like inclusions containing ubiquitin and α-synuclein. Rotenone can be administered by many routes as it crosses the blood-brain barrier due to its high lipophilic profile. Injections to the MFB cause loss of dopamine and systemic administration causes damage in the striatum and nigral proteinaceous inclusions (Betarbet et al., 2000; Ferrante, Schulz, Kowall, & Beal, 1997; Heikkila et al., 1985; Ravenstijn et al., 2008). However, most groups have noticed that only some of the animals have nigrostriatal degeneration and it does not seem to work well in other animals besides the rat. In addition, the specificity of the damage to the nigrostriatal system has been questioned and high accounts of mortality reported has caused problems in reproducibility in rotenone models of PD (Betarbet et al., 2000; Greenamyre, Cannon, Drolet, & Mastroberardino, 2010; Hoglinger et al., 2003). So far, the best working models have used repeated low dose i.p. administrations for at least 30 days, and with the use of novel delivery vehicles have reduced mortality and provided robust motor deficits, which indicates that the model could be very useful and reproducible with careful setup and optimization (Alam & Schmidt, 2002; Cannon et al., 2009).
Paraquat (N ,N ′-dimethyl-4-4′-bipiridinium) is a herbicidic compound that resembles MPP+ and has been shown to cause major toxicity to several organs, such as the lungs, liver, and kidneys. In contrast to MPP+ and despite the similarities in structure, paraquat seems to cross the blood-brain barrier, most likely through a neutral amino acid transporter, in an age-dependent manner and does not require DAT to enter dopaminergic neurons (Corasaniti, Defilippo, Rodino, Nappi, & Nistico, 1991; Richardson, Quan, Sherer, Greenamyre, & Miller, 2005; Shimizu et al., 2001). Its mechanism of causing cell death also differs from rotenone and MPP+ as it is not effective in blocking complex I. Paraquat induces apoptosis through the intrinsic mitochondrial pathway and causes major oxidative stress acting as a redox cycler and inhibiting the antioxidative action of glutathione (Day, Patel, Calavetta, Chang, & Stamler, 1999; Fei, McCormack, Di Monte, & Ethell, 2008; Richardson et al., 2005). Attempts at using it to develop PD animal models have been conflicting as some researchers have observed nigrostriatal loss of dopamine and dopaminergic neurons with deficits in motor behavior, whereas others have not observed any of these effects using similar protocols in mice (McCormack & Di Monte, 2003; McCormack et al., 2002; Thiruchelvam, Brockel, Richfield, Baggs, & Cory-Slechta, 2000).
As with proteasomal inhibitors, the presence of α-synuclein inclusions has garnered interest in pesticide models. Further studies demonstrated that, similar to lactacystin, combinations of paraquat with other compounds, such as maneb, may provide a synergistic effect and more robust lesions with motor impairments (Bastias-Candia, Di Benedetto, D'Addario, Candeletti, & Romualdi, 2015; Caputi et al., 2015).
GENETIC MODELS
Two different, albeit interconnected, types of genetic mouse models of PD have been created—GWAS-based and etiological models (http://www.informatics.jax.org/disease/DOID:14330). Several genetic variants are associated with familial early-onset PD, the major ones affecting α-synuclein and LRRK2 (with autosomal dominant inheritance) and DJ-1, PINK1, and Parkin (with autosomal recessive inheritance; see https://www.omim.org/phenotypicSeries/PS168600 for the regularly updated list of genetic variants associated with PD) (Klein & Westenberger, 2012). Naturally, after identification of these variants, attempts have been made to create mice with corresponding mutations; however, surprisingly, introducing PD-related mutations to mice resulted in only a mild neurodegeneration phenotype.
Alpha-Synuclein
The majority of transgenic mouse models focused on the expression of wild-type or mutant α-synuclein, and have been extensively reviewed elsewhere (Breger & Fuzzati Armentero, 2019; Koprich, Kalia, & Brotchie, 2017; Visanji et al., 2016). Most of these transgenic mice present very mild, if any, loss of dopaminergic neurons and many do not develop α-synuclein aggregation (Breger & Fuzzati Armentero, 2019). Interestingly, alanine to threonine substitution at position 53 (A53T) in human α-synuclein is associated with familial PD (Polymeropoulos et al., 1997), whereas mouse wild-type α-synuclein normally has threonine at position 53, which is not causing a PD phenotype. Further, a substrain of C57BL/6J mice (C57Bl/6OlaHsd) possesses a chromosomal deletion of α-synuclein locus and the animals appear phenotypically normal (Specht & Schoepfer, 2001). Thus, species-specific differences between mice and humans in sensitivity of dopaminergic neurons to α-synuclein mutations clearly exist. Recently, a novel transgenic mouse model expressing truncated α-synuclein under control of the rat TH promoter on α-synuclein-null genetic background has been created. These mice develop age-dependent loss of nigral dopaminergic neurons and striatal fibers, progressive accumulation of α-synuclein inclusions, reduced striatal dopamine content and release, and locomotor abnormalities, recapitulating key features of PD. Moreover, the authors demonstrated application of this model for pre-clinical drug development, showing that a small molecule modulator of α-synuclein aggregation rescued dopaminergic neurons and behavior phenotype in these mice (Wegrzynowicz et al., 2019).
LRRK2
Several transgenic mouse lines have been created carrying PD-associated mutations in LRRK2, but none recapitulate the Parkinsonian phenotype observed in human patients (Bichler, Lim, Zeng, & Tan, 2013; Chen et al., 2012; Li et al., 2010; Li et al., 2009; Ramonet et al., 2011; Weng et al., 2016). Bacterial artificial chromosome (BAC) transgenic mice overexpressing human LRRK2-R1441G at 5 to 10-fold relative to endogenous levels develop normally, without loss of dopaminergic neuron numbers or striatal dopaminergic innervation. Nevertheless, compared to control mice overexpressing wild-type LRRK2 at the same levels, the mice expressing LRRK2-R1441G exhibit attenuated dopamine release and axonal abnormalities, as well as reduced mobility, which, by 10-12 months of age, progresses to immobility in most mutant animals (Li et al., 2009). However, independent analysis of the same LRRK2-R1441G transgenic mouse line observed gastrointestinal dysfunction from 6 months of age, but no abnormalities in a battery of behavioral tests, no changes in learning and memory, and, contrary to the original data, no signs of akinesia (Bichler et al., 2013). The cause of the apparent discrepancy between these studies remains unclear. In line with the latter results, knockin mice having endogenous levels of LRRK2 with R1441C mutation appear normal and, while having impaired dopamine D2 receptor function, show normal striatal dopamine levels without dopaminergic neuron loss up to 2 years of age (Tong et al., 2009). Similarly, 12-month-old BAC transgenic mice with strong overexpression of mouse LRRK2-G2019S exhibit decreased striatal dopamine content, but no differences in levels, enzymatic activity, or phosphorylation state of striatal TH protein, and no loss of dopaminergic neurons. Interestingly, overexpression of wild-type mouse LRRK2 resulted in elevated striatal dopamine release, hyperactivity, and enhanced motor performance (Li et al., 2010). However, in yet another transgenic model utilizing human BAC to overexpress wild-type or LRRK2-G2019S, reduced striatal dopamine levels without neurodegeneration have been observed (Melrose et al., 2010). Similarly, reduced striatal dopamine content, fragmentation of Golgi in dopaminergic neurons, and increased microtubule polymerization, as well as motor deficits in the beam walking and rotarod tests, but no dopaminergic neuron loss have been observed in mice overexpressing human LRRK2-I2020T under control of the CMV promoter (Maekawa et al., 2012). Several other groups attempted to overexpress wild-type or mutant LRRK2 under control of CMV enhancer/PDGF-β promoter driving neuron-specific transgene expression, including dopaminergic neurons (Liu, Wang, Ma, & Wang, 2004). Transgenic mice expressing human LRRK2-R1441C and G2019S at 3-5-fold levels compared to endogenous LRRK2 exhibit age-related degeneration of dopaminergic neurons in some 19-20-month-old LRRK2-G2019S, but not in LRRK2-R1441C mutant animals, without apparent changes in striatal dopamine concentrations or behavioral phenotype (Ramonet et al., 2011). Expression of human LRRK2-G2019S caused progressive loss of nigral dopaminergic neurons and striatal terminals at 12 and 16 months of age, as well as associated motor deficits in open field and pole climbing tests (Chen et al., 2012). The same research group generated LRRK2-R1441C transgenic mice, using a similar transgenic construct and observed loss of nigral dopaminergic neurons, striatal terminals, and motor behavioral deficits in 16-month-old mice (Weng et al., 2016). However, conditional expression of human LRRK2-R1441C induced by Cre recombinase expressed in dopaminergic neurons under control of DAT promoter [DAT-Cre mice (Turiault et al., 2007)] did not induce degeneration of dopaminergic neurons and no motor deficits have been observed (Tsika et al., 2014). Tetracycline-inducible overexpression of LRRK2-G2019S also did not cause neurodegeneration but accelerated the progression of neuropathological abnormalities in A53T α-synuclein transgenic mice (Lin et al., 2009). However, these results have also been challenged by later studies failing to demonstrate the effect of LRRK2 overexpression on α-synuclein pathology (Herzig et al., 2012); the discrepancy is attributed to cell type and brain region-specific differences in the transgene expression.
DJ-1
Modeling autosomal recessive mutations in mice has not resulted in recapitulation of a Parkinsonian phenotype either. Mice with a loss-of-function deletion of DJ-1 have impaired nigrostriatal dopaminergic function, display reduced locomotor and rearing activity in the open field test at the age of 11 months, and by 5 months of age, the males had impairment in the adhesive tape removal test. However, no differences in rotarod test, no protein inclusions, and no dopaminergic neuron loss have been observed (Chen et al., 2005). Nigrostriatal dopamine system function appeared normal without any pathological changes in another DJ-1 knockout model (Chandran et al., 2008). Careful examination of dopamine dynamics and electrophysiological studies on acute striatal slices demonstrated reduced evoked dopamine overflow, impaired activation of D2 autoreceptors, and impaired long-term depression in medium spiny neurons of DJ-1 knockout mice (Goldberg et al., 2005). Interestingly, early onset unilateral degeneration of nigral dopaminergic neurons and the loss of noradrenergic neurons in the locus coeruleus have been observed in a subset of DJ-1 knockout mice backcrossed onto a C57Bl/6 genetic background; however, the molecular mechanisms explaining the susceptibility of these mice to neurodegeneration remain unclear (Rousseaux et al., 2012). Though not reproducing the neurodegeneration phenotype, DJ-1 knockout mice have been instrumental in studying the functions of the DJ-1 protein, demonstrating its involvement in scavenging mitochondrial H2O2 (Andres-Mateos et al., 2007). In line with these data, DJ-1 knockout mice have increased sensitivity to MPTP and oxidative stress (Kim et al., 2005).
PINK1
Similarly, knockout of the mitochondrial protein kinase PINK1 did not result in a major neurodegeneration phenotype, though defects in mitochondrial functions (Gispert et al., 2009) and impairments of corticostriatal long-term potentiation and long-term depression (Kitada et al., 2007) have been observed. Studies in PINK1 knockout mice demonstrated the importance of PINK1 protein for normal calcium-induced mitochondrial permeability transition and the maintenance of striatal dopamine levels and turnover. PINK1 deletion affected NF-κB signaling and increased the levels of both pro-inflammatory and anti-inflammatory cytokines after induction of peripheral inflammation (Akundi et al., 2011). Impaired gait and olfactory dysfunctions and alterations of mitochondrial fragmentation have also been demonstrated (Glasl et al., 2012). Altogether, similar to DJ-1 knockout animals, PINK1 knockout mice exhibit mildly impaired function of the nigrostriatal dopamine system and may be useful to model the prodromal PD stage preceding the development of neurodegeneration.
Parkin
Despite high initial hopes, knockout of ubiquitin ligase Parkin in mice did not result in a robust PD model (Perez & Palmiter, 2005). Nigrostriatal dopamine system deficits have been observed in Parkin knockout mice without the loss of dopaminergic neurons up to 24 months of age (Goldberg et al., 2003; Itier et al., 2003). A different Parkin knockout mouse line exhibited reduced life span, abnormal gait, accumulation of Tau, and moderate loss of dopaminergic neurons with aging (Rodriguez-Navarro et al., 2007). Interestingly, Parkin deletion resulted in the degeneration of noradrenergic neurons in the locus coeruleus and, consequently, a reduction of the noradrenaline-dependent acoustic startle response (Von Coelln et al., 2004). Impaired in vivo dopamine release without neurodegeneration has been observed in another Parkin knockout model (Oyama et al., 2010). The presence of mild impairments in synaptic plasticity affecting learning and memory in Parkin-deficient mice raised hope that these animals can be used to model early prodromal stages of PD (Rial et al., 2014). However, a careful side-by-side comparative analysis of Parkin, PINK1, DJ-1 knockout, and LRRK2-R1441G transgenic mice did not detect any difference in dopamine release between these animals and wild-type controls, suggesting that mutations in these genes are not sufficient to affect either survival or function of mouse dopaminergic neurons, at least in young adult animals (Sanchez et al., 2014). In an attempt to boost a neurodegenerative phenotype in Parkin knockout mice, these animals were crossed with other mutant models. The mice overexpressing Parkin-associated endothelin receptor-like receptor (Pael-R), shown to be a Parkin substrate, on a Parkin knockout background exhibited accumulation of Pael-R causing increased oxidative and endoplasmic reticulum (ER) stress and unfolded protein response leading to activation of ER-stress-mediated cell death pathways and age-related neurodegeneration of dopaminergic neurons (Wang et al., 2008). Similarly, deletion of Parkin combined with the overexpression of human mutant α-synuclein resulted in structural and functional abnormalities of mitochondria in neurons and glia (Schmidt et al., 2011; Stichel et al., 2007). Interestingly, a BAC transgenic model expressing C-terminal truncated human mutant Parkin (Parkin-Q311X) at physiological levels in dopaminergic neurons under control of the mouse DAT promoter have been created, aiming to test possible dominant toxic effects of this Parkin mutation. These mice exhibited progressive locomotor abnormalities in open field, beam walking, cylinder, and adhesive tape removal tests, accumulation of proteinase K-resistant α-synuclein inclusions, oxidative protein damage, and age-related loss of nigral dopaminergic neurons (Lu et al., 2009).
Parkin, PINK1, and DJ-1 proteins form a ubiquitin E3 ligase complex promoting ubiquitination and proteasomal degradation of Parkin substrates. Ubiquitination activity of this protein complex is reduced after genetic deletion of PINK1 or DJ-1 (Xiong et al., 2009). Considering these results and the absence of dopaminergic neuron degeneration in single knockout mice, triple Parkin/DJ-1/PINK1 knockout mice have been created. However, even this triple knockout did not result in degeneration of either dopaminergic neurons in the midbrain or noradrenergic neurons in the locus coeruleus at up to 24 months of age (Kitada, Tong, Gautier, & Shen, 2009).
Etiological Models
In parallel with the models based on mutations associated with PD, a number of other transgenic mouse models have been created aiming to study the effect of particular genes on survival and maintenance of dopaminergic neurons in adult and aged animals. Generally, these models have been more successful in recapitulating neurodegeneration and a PD-like behavioral phenotype. The drawback of many etiological models is that the effect of mutations of genes essential for dopaminergic neuron survival is so profound that it cannot be reversed by experimental treatments, limiting usefulness of these models for pre-clinical studies. The targeted genes were either involved in regulating crucial pro-survival cellular pathways, affected by aging and/or stress, or deregulated in PD patients. Interestingly, analysis of heterozygous deletion mutants for several genes involved in dopaminergic neuron differentiation have uncovered their role in adult and aged neurons. For example, heterozygous deletion of transcription factor Nurr1 led to decreased evoked dopamine release, depletion of striatal dopamine levels, and loss of dopaminergic neurons in aged animals (Zhang, Le, Xie, & Dani, 2012). Similarly, aged mice with heterozygous deletion of Foxa2 developed motor abnormalities and progressive asymmetric loss of nigral dopaminergic neurons (Kittappa, Chang, Awatramani, & McKay, 2007). Mice heterozygous for Engrailed1 transcription factor also showed age-related progressive degeneration of dopaminergic neuron axons in the dorsal striatum and cell bodies in the SN (Nordstroma et al., 2015). However, it cannot be excluded that haploinsufficiency for these factors already affected dopaminergic neurons at the embryonic stage and this developmental abnormality manifested itself as increased sensitivity to stress and eventual neurodegeneration later in life. To overcome this problem, tamoxifen-inducible CreERT2 recombinase was expressed under control of the DAT promoter (DAT-CreERT2) enabling selective conditional ablation of “floxed” genes in adult dopaminergic neurons (Engblom et al., 2008). Several dopaminergic neuron-specific Cre recombinase expressing mouse lines (DAT-Cre) have also been created (Backman et al., 2006; Ekstrand et al., 2007; Turiault et al., 2007; Zhuang, Masson, Gingrich, Rayport, & Hen, 2005). Despite concerns about specificity of Cre expression (Papathanou, Dumas, Pettersson, Olson, & Wallen-Mackenzie, 2019) and spontaneous tamoxifen-independent recombination (“leakiness”) of CreERT2 (Kristianto, Johnson, Zastrow, Radcliff, & Blank, 2017; Sandlesh, Juang, Safina, Higgins, & Gurova, 2018), these mouse lines have been extremely useful to study functions of multiple genes in dopaminergic neurons. For example, analysis of sonic hedgehog (Shh)/DAT-Cre mice revealed that Shh expression in dopaminergic neurons is essential for their maintenance in the adult animals, as the mutant mice exhibited neurodegeneration, locomotor, and gait abnormalities (Gonzalez-Reyes et al., 2012). Similarly, conditional ablation of Foxa1 and Foxa2 in the adult Foxa1/Foxa2/DAT-CreERT2 mice resulted in locomotor impairments, reduced striatal dopamine, and degeneration of dopaminergic neurons in aged animals (Domanskyi et al., 2014). In addition, deletion of Nurr1 in mature dopaminergic neurons resulted in reduced striatal dopamine content, axonal dystrophy, and locomotor phenotype, but was not associated with neurodegeneration (Kadkhodaei et al., 2013). Severe downregulation of the vesicular monoamine transporter 2 (VMAT2) in dopaminergic neurons demonstrated its importance for adult dopaminergic neuron maintenance. Hypomorphic mice expressing very low levels of VMAT2 have decreased striatal dopamine levels, elevated oxidative stress, locomotor impairments, and neurodegeneration at old age. Importantly, these mice also show α-synuclein accumulation in nigral dopaminergic neurons (Caudle et al., 2007).
Mitochondrial dysfunctions have been associated with PD development and progression (Park, Davis, & Sue, 2018). MPTP, a widely used toxin for PD modeling, causes an effective inhibition of complex I respiration in dopaminergic neuron mitochondria (Meredith & Rademacher, 2011; Schober, 2004). In attempts to create a robust and reliable genetic PD model, several genes important for mitochondrial function have been targeted. Indeed, dopaminergic neuron-specific deletion of Cox10 (Cox10/DAT-Cre mice), a protein essential for functional maturation of cytochrome c oxidase, caused severe degeneration of dopaminergic neuron axons and cell bodies, neuroinflammation, and depletion of striatal dopamine with robust and progressive motor behavioral deficits, resembling advanced PD. Importantly, motor impairments could be reverted by L-DOPA or pioglitazone treatment, demonstrating usefulness of this PD model for pre-clinical drug testing (Pinto et al., 2016). However, not all genetic mutations affecting mitochondria resulted in a neurodegeneration phenotype. For example, deletion of Mitofusin 2, which regulates fusion of outer mitochondrial membrane, in dopaminergic neurons (Mfn2/DAT-Cre) caused mitochondrial fragmentation and respiratory chain deficiency leading to the loss of dopaminergic axonal projections in the striatum with severe depletion of striatal dopamine levels, but no degeneration of nigral dopaminergic neurons (Lee et al., 2012). Surprisingly, genetic inhibition of mitochondrial complex I achieved by deletion of one of its subunits, Ndufs4 , in dopaminergic neurons (Ndufs4/DAT-Cre) did not lead to the loss of nigral dopaminergic neurons or increased susceptibility to MPTP; however, these mice had reduced striatal dopamine concentration and accumulation of phosphorylated α-synuclein in nigral dopaminergic neurons (Kim et al., 2015). An interesting approach to induce mitochondrial DNA damage was achieved by inducible dopaminergic neuron-specific expression of Pst I endonuclease targeted to the mitochondria, which caused double-strand breaks in the mtDNA, depleting its levels, and leading to the formation of large deletions. These PD-mito-PstI mice exhibited a progressive age-dependent degeneration of nigral dopaminergic neurons, depletion of striatal dopamine, and motor behavioral abnormalities, which could be reversed with L-DOPA treatment (Pickrell, Pinto, Hida, & Moraes, 2011). But perhaps the most successful mitochondrial genetic PD model is the MitoPark mouse, carrying conditional deletion of mitochondrial transcription factor A (Tfam) in dopaminergic neurons (Tfam/DAT-Cre). These mice have dramatically reduced expression of genes encoded by mitochondrial DNA, such as cytochrome c oxidase, causing respiratory chain deficiency which leads to the adult-onset progressive locomotor deficits, loss of dopaminergic neurons and their axonal projections, and a behavioral phenotype. The MitoPark mouse is one of the first genetic models recapitulating most of the key PD features, as these animals also accumulate intraneuronal inclusions containing mitochondrial proteins and membrane components (Ekstrand & Galter, 2009; Ekstrand et al., 2007; Galter et al., 2010). Unfortunately, the inclusions do not contain α-synuclein, and for that reason the model was criticized as not faithfully reproducing Lewy body formation (Potashkin, Blume, & Runkle, 2010). However, a recent study demonstrated that, in addition to α-synuclein, Lewy bodies also contain membranes originating from vesicles and fragmented mitochondria (Shahmoradian et al., 2019). Moreover, MitoPark mice recapitulate the gastrointestinal dysfunction and gut microbiome changes characteristic of early PD (Ghaisas et al., 2019). Therefore, perhaps, the utility of the MitoPark mice as a robust and reproducible genetic PD model with a gradually progressing dopaminergic neurodegeneration phenotype and protein inclusions is much higher than recognized. Importantly, behavioral abnormalities in these mice can be alleviated with L-DOPA, as well as small molecule drugs, demonstrating that the MitoPark mouse PD model can be successfully used for modeling L-DOPA-induced dyskinesia or utilized for pre-clinical testing of novel PD therapies, including cell transplantation (Chen et al., 2018; Ekstrand et al., 2007; Langley et al., 2017; Shan et al., 2015; Zhang, Granholm, et al., 2012).
Nucleolar disruption in dopaminergic neurons has been observed in post-mortem midbrain sections of PD patients. The hypothesis that age-related nucleolar stress and deregulation of ribosomal RNA synthesis can predispose dopaminergic neurons to degeneration in PD has been directly tested in TIF-IA/DAT-CreERT2 mice carrying dopaminergic neuron-specific tamoxifen-inducible deletion of TIF-IA (also known as RNA polymerase I-specific transcription initiation factor, RRN3). The mice exhibited progressive degeneration of dopaminergic neurons, reduced striatal dopamine levels, and behavioral abnormalities (Rieker et al., 2011). In contrast to PD patients and toxin-induced mouse PD models, nucleolar integrity was not affected by DJ-1/PINK1 double knockout, highlighting the value of the TIF-IA conditional knockout model to study PD-related nucleolar dysfunction (Evsyukov et al., 2017). The utility of TIF-IA/DAT-CreET2 mice for pre-clinical drug testing has recently been proven by the study showing a neuroprotective effect of reboxetine, a selective noradrenaline reuptake inhibitor, on dopaminergic neurons (Kreiner et al., 2019). These mice have also been used to demonstrate the neuroprotective outcome of Akt pathway activation in adult dopaminergic neurons (Domanskyi et al., 2011).
Last but not least, the importance of microRNA biogenesis for dopaminergic neuron survival and function has been demonstrated in a series of studies in mice with conditional knockout of Dicer, a crucial enzyme for generation of mature microRNAs (Chmielarz et al., 2017; Kim et al., 2007; Pang et al., 2014). The Dicer/DAT-CreERT2 mice show progressive loss of dopaminergic neurons and striatal dopamine, leading to strongly reduced activity and impaired performance in the open field and rotarod tests, respectively (Chmielarz et al., 2017). Interestingly, even the heterozygous inducible deletion of Dicer in dopaminergic neurons led to reduced concentrations of striatal dopamine and its metabolites (Chmielarz et al., 2017), demonstrating that age-related changes in Dicer and microRNA levels observed in PD patients (Briggs et al., 2015) can affect function of dopaminergic neurons and increase their vulnerability to stress, contributing to neurodegeneration in PD. In line with this hypothesis, pharmacological stimulation of microRNA biogenesis promoted survival of dopaminergic neurons (Chmielarz et al., 2017). This is another example illustrating how the study of an etiological PD model led to the development of new drug treatments.
In summary, the GWAS-based mouse PD models (except certain α-synuclein transgenic mice) have been surprisingly inefficient in recapitulating human PD features. Why is it that genetic mutations causing PD in humans do not recapitulate the disease in the mouse? Clearly, careful analysis of species-specific differences between mice and humans, such as the presence of neuromelanin and differences in dopamine metabolism will be crucial for understanding differential vulnerability of dopaminergic neurons in mouse models versus PD patients. Indeed, a recent study demonstrated that increase in either α-synuclein or dopamine levels in mouse DJ-1 knockout dopaminergic neurons promoted their degeneration and recapitulated human dopaminergic neuron pathology (Burbulla et al., 2017). In comparison to GWAS-based mutants, etiological PD models are much more successful in recapitulating key PD features and appear to be more useful for pre-clinical studies of novel therapies. However, in order to be relevant for PD, these models need to target genes and pathways showing age- and/or disease-related changes in PD patients.
ALPHA-SYNUCLEIN OVEREXPRESSION MODELS
Adeno-Associated Virus-Alpha-Synuclein Model
Using adeno-associated virus (AAV) to overexpress α-synuclein in animals is a popular model amongst PD researchers (Albert, Voutilainen, Domanskyi, & Airavaara, 2017). Most commonly, AAV carrying the human α-synuclein transgene is stereotaxically injected unilaterally above the SNpc to overexpress the α-synuclein protein in the area. Both wild-type and mutant forms (ex. A53T) of α-synuclein have been used for this purpose (Decressac, Mattsson, Lundblad, Weikop, & Bjorklund, 2012; Febbraro et al., 2013) and although the wild-type form is more often utilized, there have been effects with overexpression of the mutated α-synuclein protein as well (Koprich et al., 2017). The model produces α-synuclein protein expression in the dopaminergic neurons of the nigrostriatal tract which can cause loss of TH expression and unilateral motor deficits (Decressac et al., 2013; Decressac, Mattsson, & Bjorklund, 2012). While loss of TH in the SNpc is common in this model, the outcome of other measures often used in the field of animal models of PD such as loss of TH expression in the striatum, dopamine content levels, and certain motor phenotypes are less clearly observed or not shown at all (Albert et al., 2017).
One may attribute different outcomes in this model to the variety of serotypes, promoters, wild-type versus mutant α-synuclein, number of particles injected, and time points used—and this is the case. However, it is also a pertinent question as to whether the model in general recapitulates sporadic PD as it purports to, since in the AAV-α-synuclein model in rats SNCA (gene encoding α-synuclein) mRNA is increased (Decressac, Mattsson, Lundblad, et al., 2012), whereas in PD patients the mRNA is actually decreased (Kingsbury et al., 2004). A study confirmed that this is indeed the case in the model, that SNCA mRNA is increased in rats and in patients decreased (Su et al., 2017), concluding that this is not necessarily the most useful model of sporadic PD.
In addition to its lack of validity as a model of the sporadic disease, we have found that the AAV-α-synuclein model is not ideal for therapy testing. In our hands, we were able to repeat published data in terms of TH downregulation and behavioral deficits on the cylinder test (Albert et al., 2019); however, there was large variation between the animals and little to no correlation between amount of α-synuclein present, TH, and behavior. Testing therapies on this model in this manner, by measuring typical outcome measures used in the field, is therefore not appropriate. One should use other outcome measures such as different behavioral assays (Gombash et al., 2013), or a way to measure dopaminergic deficits that does not use immunohistochemistry such as SPECT/CT, a combination of single-photon emission computed tomography and computed tomography with radioligands for DAT, so that animals can be checked for a lesion during the experiment (Back et al., 2013). Nevertheless, the AAV-α-synuclein model can be a good model to evaluate compounds that are aiming to enhance clearance of α-synuclein, or its aggregated forms, and in fact for this it is rather valuable (Chmielarz et al., 2019). However, at the moment many of the outcome measures used in the model are the same as for toxin models.
While it is possible to use different measurements and techniques to make the AAV-α-synuclein more successful, this overlooks a key component of any model: the control. In an AAV disease model, as with any AAV, a vector that overexpresses a different protein to control for unspecific effects of the relevant disease vector is needed. The protein most often used in AAV studies is GFP. The GFP used in biology studies is derived from a species of jellyfish (Ormo et al., 1996). In addition to the fact that it is not derived from mammals, it has been shown to be toxic to dopaminergic neurons in the SNpc of rats when overexpressed at high levels via AAV (R. L. Klein et al., 2006). Further, when injected to the striatum of non-human primates, AAV9-eGFP showed astrogliosis and microgliosis in the transduced area, and animals that received the vector to the cisterna magna showed behavioral deficits (Samaranch et al., 2014). However, in the same study, non-human primates receiving AAV9-hAADC (aromatic L-amino acid decarboxylase derived from human) showed no such gliosis or behavioral phenotype. In relation to AAV-α-synuclein, experiments where eGFP is expressed at similar levels to the α-synuclein resulted in loss of TH-positive cells in the SNpc in rats (Koprich et al., 2011; Landeck, Buck, & Kirik, 2016). We observed a similar effect (Albert et al., 2019), where there was a loss of TH in the animals injected with AAV-α-synuclein and AAV-eGFP over multiple experiments and conditions. Although this is likely a loss of TH phenotype since we observed the same phenomenon with AAV-DIO-mCherry which in our experiments does not express protein since the animals lack Cre recombinase, and also there was no global cell loss as evident from Nissl staining. Therefore, since TH is known to be a highly regulated enzyme in dopamine synthesis, we and others would recommend VMAT2 as a marker of dopaminergic neurons in these studies (Decressac et al., 2011). And while it is well-known in the field that decreasing the titer of AAV-GFP to be lower than AAV-α-synuclein results in little to no toxicity, we would ask if this is a relevant control. If unspecific protein expression is also causing cell loss similarly to the protein of interest, then the only thing separating the AAV-α-synuclein model from a classic toxin model is the presence of high, non-physiological levels of α-synuclein protein. One remedy for this is to use proteins that are derived from mammals and form oligomers but are not prone to misfolding, such as actin or collagen, as controls.
AAV-α-synuclein is a popular model amongst PD researchers; however, setting it up needs caution and several modifications from the current literature for it to be a useful model. If setting it up for the first time, outcome measures and controls need to be carefully considered. And in the end, this may not remove the variation between animals or the lack of correlation between outcome measures—making it difficult to test new therapies. Additionally, it is questionable whether the AAV-α-synuclein model in rats recapitulates the sporadic form of PD.
Preformed Alpha-Synuclein Fibrils Model
As discussed above, several studies using different viral vectors were reported to result in neuronal cell death in the SNpc, decreased dopamine release in striatum and motor deficits (Albert et al., 2017; Visanji et al., 2016). However, overexpression of α-synuclein relies on very high non-physiological levels of protein, which cause cell death due to protein buildup, and, therefore, might not mimic sporadic PD pathology. In addition, α-synuclein overexpression does not always lead to formation of insoluble α-synuclein-positive inclusions. As an alternative, a new model using the misfolded insoluble form of α-synuclein was recently developed and quickly became popular. This model utilizes insoluble species of α-synuclein, which, when injected to the brain or applied to in vitro cultures, recruit endogenous α-synuclein and form aggregates in the cell body and neurites, which are capable of spreading from one neuron to another (Volpicelli-Daley et al., 2011). Any effect observed in this model fully depends on the ability of recombinant α-synuclein to recruit endogenous α-synuclein, since no Lewy body-like pathology was observed in α-synuclein knockout neurons after preformed fibrils (PFFs) inoculation. Importantly, fibrils recruit specifically α-synuclein: solubility of β-synuclein was shown to be unaltered (Luk, Kehm, Carroll, et al., 2012; Luk, Kehm, Zhang, et al., 2012).
The first report demonstrating that exogenous insoluble α-synuclein fibrils are capable of modeling Lewy body-like pathology was published by Luk et al. (2009). They showed that purified human wild-type α-synuclein fibrils recruit endogenous α-synuclein causing its aggregation and formation of Lewy body-like inclusions positive for key Lewy body markers observed in PD patients, such as ubiquitin and pS129-α-synuclein immunoreactivity. A later study showed that fibrils are internalized by neurons and capable of propagating in the cells, causing disruption of normal neuronal function and eventual death in vitro. After these initial reports, the first in vivo study followed, demonstrating that PFFs are capable to induce Lewy body-like pathology in wild-type mice (Luk, Kehm, Carroll, et al., 2012). This study demonstrated that a single intrastriatal PFFs injection caused extensive spread of α-synuclein inclusions in a time-dependent manner, involving striatum, cortex, olfactory bulb, thalamus, and importantly SNpc. In addition, Lewy body-like pathology was accompanied by decreased striatal dopamine content at 3 months and loss of TH-positive neurons in the SNpc at 6 months, as well as impairment of motor behavior. Several other groups were using a similar approach with similar outcomes. The Abdelmotilib et al. (2017) study reported vast expansion of Lewy body-like pathology in the cortex and SNpc and dopamine neuron loss at 6 months after intrastriatal PFFs injection in mice and rats. Importantly, this study also demonstrated that the effect can be animal strain-dependent. Karampetsou et al. (2017) demonstrated Lewy body-like pathology spread to SNpc, dopamine neuron loss, and some motor deficits in mice as early as 2 months after injection of phosphorylated at S129 PFFs into the striatum. Similar results were demonstrated in marmosets and cynomolgus monkeys injected with PFFs into striatum. It was reported that Lewy body-like inclusions were generated in TH-positive neurons in the SNpc, coinciding with significant neuronal loss (Chu et al., 2019; Shimozawa et al., 2017).
Additionally, other sites of PFF inoculation were also reported. Studies in PD brains suggest that α-synuclein pathology spreads to the brainstem from the olfactory bulb and/or from gastrointestinal tract via enteric vagus nerve and then spreads further with disease progression. Rey et al. (2016) created a mouse model of prodromal PD by injecting fibrillar α-synuclein into the olfactory bulb. After 12 months, pS129-positive inclusions were detected throughout the brain in connected areas, including the SNpc. However, there was no further progression of Lewy body-like pathology at 23 months when pS129 was propagation studied after injecting PFFs into the mouse gastric wall (Rey et al., 2018; Uemura et al., 2018). The study demonstrated formation of pS129-positive aggregates in the dorsal motor nucleus of the vagus nerve, which could be prevented by vagotomy. However, the pathology did not spread beyond the dorsal nucleus up to 12 months and the number of pS129-positive neurons decreased over time. A similar model in rats and non-human primates also only showed minor Lewy body-like pathology in rats at 1 month, which was gone over time despite persistent pathology in the enteric nervous system. Nor was Lewy body-like pathology (Rey et al., 2018; Uemura et al., 2018) detected in non-human primates at 12 months, despite extensive spread of α-synuclein pathology in the enteric nervous system (Manfredsson et al., 2018).
Although dopaminergic neurons are the primary group of cells affected in PD, Lewy bodies are also found in other brain areas including the hippocampus and cortex. It is proposed that α-synuclein aggregation in these areas may be related to the development of cognitive deficits observed in some PD patients. Therefore, induction of Lewy body-like pathology with PFFs in hippocampal and cortical neurons was used as a model to study this aspect of PD. For example, Nouraei et al. (2018) injected PFFs into mice hippocampus and caused formation of α-synuclein-positive inclusions, which were insufficient to cause hippocampal cell death, but their expansion was negatively correlated with memory capacity. Blumenstock et al. (2017) reported that α-synuclein accumulation induced by intrastriatal injection of PFFs caused dendritic spine loss in the mouse somatosensory cortex.
Typically, prion diseases are distinguished by the presence of various protein strains that can exhibit differences in various properties, such as cell binding and penetration, propensity to induce aggregation and neurotoxicity (Bousset et al., 2013). Different species of α-synuclein were proposed to cause different synucleinopathies, and therefore, were used as a model of α-synuclein pathology. α-synuclein monomers and oligomers were shown to cause protein aggregation neither in vitro nor in vivo despite spreading more efficiently than insoluble α-synuclein species (Gribaudo et al., 2019; Luk et al., 2009; Peelaerts et al., 2015). Peelaerts et al. (2015) also reported that another insoluble form of recombinant α-synuclein, named ribbons, cause rather Lewy neurite-like filamentous phenotype, while fibrils demonstrated the highest neurotoxic potential. To contrast, Gribaudo et al. (2019) assessing propagation of fibrils and ribbons in human-derived neurons demonstrated that both ribbons and fibrils form similar inclusions that persist over time and are transferred between neurons. Both strains have no effect on cell viability or morphology, but alterations in Ca2+ homeostasis with progression of α-synuclein aggregation and altered mitochondrial morphology were recorded. However, it is important to keep in mind that currently there is no evidence of different misfolded α-synuclein species in humans (Bousset et al., 2013).
Some studies combined synthetic α-synuclein with α-synuclein overexpression (either transgenic mice or viral overexpression). For example, Luk, Kehm, Zhang, et al. (2012) demonstrated that injection of exogenous α-synuclein into the striatum of transgenic mice expressing human α-synuclein with A53T mutation accelerated development of α-synuclein pathology and significantly reduced survival. Thakur et al. (2017) reported that combining PFFs with AAV-mediated α-synuclein overexpression in the SNpc results in more rapid development of Lewy body-like pathology in rats, accompanied by dopaminergic neuron loss, decreased dopamine levels, and impaired motor behavior. AAV-mediated overexpression of α-synuclein in rat prefrontal cortex combined with PFFs seeding results in extensive α-synuclein aggregation pathology and significant impairment of cognitive functions, such as memory, attention, and inhibitory control (Espa et al., 2019). Along with these studies, α-synuclein pathology development was accelerated by combining AAV-mediated α-synuclein overexpression and α-synuclein insoluble species (both fibrils and ribbons) inoculation in mice. Combined treatment increased the number of cells with α-synuclein-positive inclusions and increased TH-positive cell loss (while none caused neuronal death by themselves). Interestingly, combination of AAV-α-synuclein and ribbons also caused sparse α-synuclein aggregates in oligodendrocytes, raising further questions about α-synuclein species-specific toxicity (Peelaerts et al., 2015).
In conclusion, artificial insoluble α-synuclein strains persist as a valuable alternative to the viral vector-induced α-synuclein pathology model. However, despite the fact that the phenotype generated by this model develops much slower than in viral overexpression or toxin models, it is still faster than in clinical α-synuclein pathology which is believed to take approximately 5-10 years. Therefore, data interpretation from this artificial model should be taken with caution, since they might not reflect the events of the clinical disease (Okuzumi et al., 2018).
CELLULAR MODELS OF DOPAMINERGIC DEGENERATION AND PROTEIN AGGREGATION
Animals are currently the only way we can model complex interactions between different cell types and structures in the brain. However, while being simplified, in vitro cellular models offer advantages in speed, cost, ease of manipulation, and decreased animal use (Kepp, Galluzzi, Lipinski, Yuan, & Kroemer, 2011). Cellular models are ideally suited for the investigation of intracellular mechanisms underlying pathological conditions and screening of protective compounds (Falkenburger, Saridaki, & Dinter, 2016; Kepp et al., 2011; Lopes, Bristot, da Motta, Parsons, & Klamt, 2017). Moreover, with current advances in automated microscopy and the advent of machine learning-based image analysis, in vitro cellular models are becoming capable of delivering multimodal data at an unprecedented scale (Boutros, Heigwer, & Laufer, 2015). Below, we will briefly describe the most commonly utilized cell types and methodology for modeling PD in vitro.
Cells used in Modeling PD Pathology In Vitro
Cell lines like SH-SY5Y, PC12, MN9D, and CSM14.1 recapitulate some markers of dopaminergic neurons (Falkenburger & Schulz, 2006) such as the presence of dopamine, DAT, TH, and D2R. Expression levels of these proteins are usually low unless the cells are further differentiated. The procedure of differentiation can vary but often depends on treatment with neurotrophic factors like BDNF, GDNF or NGF, and retinoic acid. The disadvantages of cell line differentiation are costs, time, and variability between and even within labs. Still, obtained cells will only superficially resemble neurons and usually do not recapitulate one of the hallmarks of dopaminergic neurons—spontaneous peacemaking. Since our understanding of PD etiology is limited (Surmeier, Obeso, & Halliday, 2017), we cannot purposely recapitulate all pathological processes leading to neuronal degeneration in this disease. Therefore, it is tenable to utilize models recapitulating physiological properties of dopaminergic neurons, assuming that they will best recapitulate pathological processes in PD. Recent advancements in using cell lines for modeling PD have come in the development of Lund human mesencephalic (LUHMES) cells (Scholz et al., 2011). Differentiated LUHMES cells show a more neuronal phenotype and might exhibit electrophysiological properties resembling dopaminergic neurons, including spontaneous activity (Scholz et al., 2011). However, their disadvantages are much higher difficulty and cost of the culture.
The main advantage of using cell lines is the possibility to acquire a virtually unlimited amount of relatively homogeneous cells. This is most important when employing assays relying on measurements of bulk signal from entire culture wells (like MTT or LDH assays) and/or for acquiring of protein and RNA samples for biochemical studies. However, many measurements can now be done on a per-cell basis utilizing fluorescent dyes, immunofluorescent staining, and genetically encoded reporters (Delenclos et al., 2019; Falkenburger et al., 2016; Kepp et al., 2011). These advancements come from the application of automated microscopy capable of scanning 96- or 384-multiwell plates within hours or even minutes. Moreover, quantification of obtained data can be automatized with available open-source software (McQuin et al., 2018) or custom algorithms (Kraus & Frey, 2016).
Automated microscopy is ideally suited for measurements in primary mesencephalic neuronal cultures. Such cultures usually contain 5%-10% of bona fide dopaminergic neurons (Chmielarz et al., 2017; Planken, Porokuokka, Hanninen, Tuominen, & Andressoo, 2010). These cells, albeit isolated from embryos, mature in culture after several days, and exhibit all the main characteristics of neuronal cells and specifically of dopaminergic neurons. They grow large axonal trees, release and uptake dopamine, and show spontaneous peacemaking linked with Ca2+ fluctuations.
Additionally, primary dopaminergic neurons are exceptionally vulnerable to insults used to model PD, further confirming their relevance in modeling the disease. Utilizing early post-natal cultures, it is even possible to separately culture dopaminergic neurons from the substantia nigra and the ventral tegmental area (Sulzer, Trudeau, & Rayport, 2008). The disadvantage of primary cultures of dopaminergic neurons is that isolation of RNA or protein is not possible without an additional sorting step, and efficient gene transfer requires the use of lentiviral vectors. Nonetheless, primary cultures remain probably the best option for experiments on dopaminergic neurons, rather than dopamine-neuron resembling cells, bearing in mind that there might be differences due to animal origin of these cells.
A feasible option to study PD in vitro on human dopaminergic neurons came recently with the advent of induced pluripotent stem cells (iPSC) and advancement in protocols of obtaining iPSC-derived dopaminergic neurons (Chambers et al., 2009). It is now possible to get relatively highly enriched populations of iPSC derived cells showing all major characteristics of dopaminergic neurons (Burbulla et al., 2017). These cells give hope for increased translational relevance since they display vulnerabilities not observed in mouse dopaminergic neurons (Burbulla et al., 2017) and excitingly, can be derived from PD patients’ biopsies. IPSC-derived dopaminergic neurons will probably be the ultimate in vitro cellular model for PD. However, there are still many hurdles to overcome before their widespread use. They are extremely laborious and expensive to obtain and maintain, requiring highly trained personnel. Moreover, variability between different batches of differentiated cells can be high, although the ability to generate isogenic controls with gene editing is a very elegant way to obtain control cells.
Induction of Pathology In Vitro
Modeling dopaminergic neuron degeneration in vitro utilizes largely the same methods as described in previous paragraphs. Neurotoxins, mostly 6-OHDA and MPTP, have been commonly utilized, as well as rotenone and lactacystin (Falkenburger & Schulz, 2006). Of significant note is that specificity of 6-OHDA and MPTP largely depends on specific uptake, and hence, the expression of DAT, which can vary between batches of differentiated cell lines, and therefore, might affect the obtained results. In addition, screened compounds might affect DAT activity giving false-positive results (Falkenburger & Schulz, 2006). Moreover, the doses of toxin used can have a major impact on the study outcome since it was demonstrated that upon low toxin doses dopaminergic neurons in vitro die by apoptosis. In contrast, higher doses induce necrosis (Venderova & Park, 2012). This fact raises some concerns about the relevance of toxin-induced cell death to the demise of human dopaminergic neurons in PD. In primary cultures and for iPSC-derived cells, neuronal death can be assessed by directly quantifying surviving dopaminergic neurons using, e.g., TH immunostaining, and additionally investigated by specific cell death assays (Kepp et al., 2011). For cell lines of higher homogeneity bulk assays such as LDH release or the MTT assay can be used (Kepp et al., 2011).
Aggregation and accumulation of α-synuclein in vitro can be modeled with overexpression or α-synuclein PFFs, as described in previous paragraphs. Cell lines generally do not express high levels of endogenous α-synuclein, not surprisingly since it is a protein mainly involved in synaptic function. Therefore, cell lines usually require overexpression of α-synuclein to observe aggregation, leaving some concerns about physiological validity (Delenclos et al., 2019; Falkenburger et al., 2016). In contrast, robust aggregation of endogenous α-synuclein can be observed in primary neuronal cultures (Volpicelli-Daley, Luk, & Lee, 2014) upon addition of α-synuclein fibrils. Induction of this pathology is more robust in more mature neuronal cultures, probably due to a higher number of connections formed, and thus, higher α-synuclein expression (Volpicelli-Daley et al., 2014). For iPSC-derived neurons, induction of α-synuclein aggregation with fibrils might require a prolonged period of culture since their maturation is slower. Cell death in in vitro α-synuclein aggregation models is not very pronounced (Volpicelli-Daley et al., 2014). However, primary cultures are perfectly suited to observe the sequence of events leading to α-synuclein aggregation and for screening of compounds targeting α-synuclein, since α-synuclein aggregation can be easily quantified after immunofluorescent staining.
MORPHOMETRIC ANALYSIS IN PD MODELS
Immunohistochemistry
Analysis of the lesion, regardless of used toxin, is usually done by staining the free-floating or paraffin sections cut from the animals’ midbrain for dopaminergic neuron markers, most commonly TH, DAT, and/or VMAT2 (Kirik et al., 1998; Penttinen, Parkkinen, Blom, et al., 2018). The visualization is usually achieved either by colorimetric or fluorescent tags. Colorimetric, such as diaminobenzidine (DAB), does not require activation with specific light sources and is, therefore, best when one does not have fluorescence microscopes or scanners available. However, using infrared secondary antibodies can provide greater specificity and linearity, especially when brain tissue often has autofluorescence at shorter wavelengths. Both colorimetric (DAB) and immunofluorescent stainings (AF680 and AF800) have been used in rat brain sections when analyzing 6-OHDA lesions and the variations in optical density measurements were similar (Penttinen, Parkkinen, Blom, et al., 2018). Another advantage of using infrared wavelengths combined with whole slide scanners is multiplexing from the same sample, as colorimetric has fewer options for dual or triple stainings.
Quantification of Dopaminergic Neurites and Cell Bodies
Stereology with the optical fractionator method and unbiased counting rules has been the backbone of cell counting for the past few decades (West, Slomianka, & Gundersen, 1991). Stereology, however, is time-consuming and often requires a specialized microscope and software for efficient and unbiased counting. New computational methods have enhanced counting, e.g., implementing MatLab in calculations provide similar results as obtained with the optical fractionator unbiased counting (Penttinen et al., 2016). However, this still requires manual labor, and newer methods aim to automatize counting. The most recent advances use advanced AI algorithms, such as convoluted neural network (CNN)-based algorithms for counting dopaminergic neurons from images (Penttinen, Parkkinen, Blom, et al., 2018). Counting achieved with the CNN correlates highly with counting done by stereology and offers a workload reducing option for counting cells.
Average optical density measurements have been used in quantifying striatal innervation of dopaminergic neurons in animal models of PD for the past few decades (Burke, Cadet, Kent, Karanas, & Jackson-Lewis, 1990). Dopaminergic neurites from midbrain sections are labelled with their respective markers as described in the “immunohistochemistry” paragraph of this article, and the striatal sections are imaged with microscopes or scanners. From the photomicrographs, the optical densitometric measurements of the stainings are done with various image analysis software, such as ImageJ (or FIJI), ImagePro or e.g., Olympus software when implementing IR-staining (Penttinen, Parkkinen, Blom, et al., 2018). In unilateral models, the measurements are done from both hemispheres to assess the size of the lesion by comparing the lesioned side to the control side and subtracting the background stain, usually measured from the corpus callosum as there are no TH positive fibers there.
FUTURE PERSPECTIVES
In academic research, we have a reproducibility crisis and it has been estimated that most biomedical academic work cannot be reproduced (Eisner, 2018). In addition, higher impact factor of journals correlates with poorer reproducibility of studies. It is the purpose of academic science to do innovative research developing completely new concepts, but nonetheless we need to have integrity, especially principal investigators, and pass this along to young researchers. It cannot be that we force PhD students or post-doctoral scientists to prove that a concept or treatment is working just so that they can graduate, get the next job, and simultaneously make the laboratory look prosperous. One way to tackle this problem and to increase the quality of experimental PD research is by following the example of the stroke field, namely, develop and adhere to a set of guidelines for conducting pre-clinical studies on PD models. We could implement Stroke Therapy Academic Industry Roundtable (STAIR)-like criteria for PD if both academic and industry experts come together. The other way to solve the problem is to promote hypothesis-driven research. If the research results are important to understand basic biology of the human dopaminergic system, it does not matter whether they are negative or positive in terms of developing new treatments and moving the field forward.
As reviewed above, in many cases the originally promising results of multiple animal PD models have not been reproduced in subsequent studies, both on behavioral and cellular levels. Seemingly easy to perform behavioral tests are especially sensitive to a variety of factors; critically, sufficient number of animals per experimental group should be used (Hanell & Marklund, 2014). Journals and their editors have an important role in improving the reproducibility of experimental PD research. Journals disseminate the results to other researchers and they need to take more responsibility for this issue. There are some journals that have done this already and taken the reproducibility of published data as their key area to improve, and implemented a statistical editor to review the statistics, experimental designs, and data presentation. Students and researchers at all levels should improve their knowledge and educate more on biostatistics. These aspects do not only concern PD research, but most preclinical and biological studies as well.
It is important to understand what the current state of α-synuclein biology is. For example, while many studies show that α-synuclein is a disordered protein, these are based on experiments where α-synuclein is produced in E. coli where it is well-known that mammalian proteins do not fold similarly as they do in mammalian cells. Whether endogenous α-synuclein is monomeric or oligomeric still needs to be clarified–and in relation to this, agreement on nomenclature is still lacking. One should be clear when discussing so-called “toxic” species of α-synuclein, does it refer to α-synuclein that is causing cell death, as this appears to not always be the case, and whether this oligomeric species is endogenous protein or it is aggregated. Authors should be clear when discussing these topics.
It is rather clear that proteostasis problems and aggregated α-synuclein have a role in the disease progression. Decreasing α-synucleinopathy and aggregated α-synuclein is, thus, a very important aspect of developing new therapies for PD. Since α-synuclein is also found in blood and may reflect the concentration found in the brain, what is clearly needed is quantifiable measures for clinical outcome. We need PD biomarkers that can be quantified in a linear manner and that predict the outcome of the disease onset or progression, and moreover, the same outcome should be able to be measured from disease models and with reporters in cell cultures as well, demonstrating that it has a biological basis.
For GWAS found genes there are numerous possibilities to affect dopaminergic neurodegeneration than having a singular cause for degeneration. Past studies in mice have shown that these genes alone are not the only cause for the PD phenotype resulting from degeneration of nigrostriatal dopaminergic neurons. In the future, it would be important to study the basic biology of these genes and corresponding proteins in the concept of human dopaminergic neurons, and, more importantly what are the effects of mutation at the neurovascular unit.
Recent progress in the generation of stem cell-derived midbrain dopaminergic progenitors essentially solved the problem of human cell availability and ethical concerns, enabling studies of dopaminergic neurons derived from human stem cells in culture (Grealish et al., 2014; Nolbrant, Heuer, Parmar, & Kirkeby, 2017). The research focus may be shifting towards studying possible neuroprotective treatments in human dopaminergic neurons, which offer alternative and, possibly, a better option to model PD. Indeed, dopaminergic neurons differentiated from both familial and idiopathic PD patient-derived induced pluripotent stem (iPS) cells successfully recapitulate increased stress sensitivity, mitochondrial and synaptic dysfunctions, abnormal neuronal morphology, and pathological protein aggregation [reviewed in Beevers, Caffrey, and Wade-Martins (2013); Li, Jiang, Zhang, and Feng (2018); and Mishima, Fujioka, Fukae, Yuasa-Kawada, and Tsuboi (2018)]. However, while differentiated cells acquire a dopaminergic neuron phenotype, they are essentially embryonic or early postnatal (La Manno et al., 2016), and, though more mature neurons might be possible to obtain by purification (Sandor et al., 2017), iPS cell-derived dopaminergic neurons do not fully recapitulate the properties of the adult nigral dopaminergic neurons degenerating in PD patients.
Midbrain organoids can potentially offer a better model with the additional advantage of recapitulating the interaction between glia cells and neurons (Smits et al., 2019). However, the protocols for robust and reproducible differentiation of midbrain organoids suitable for discovery and testing of new neuroprotective treatments are not yet developed. Though it has recently been demonstrated that human organoids can be successfully transplanted to the adult mouse brain (Mansour et al., 2018), human organoids do not offer a possibility to study the effects of experimental neurorestorative treatments on a motor behavior phenotype.
Development of humanized models based on transplantation of human midbrain neuron precursors to the mice in which the endogenous dopaminergic system is severely lesioned appears to be a promising option for PD modeling. Having human dopaminergic neurons in the brain with correct cells surrounding them is an approach that should be used in future studies. One can even have human dopaminergic neurons together with human astrocytes. After transplantation to the midbrain, a proportion of human embryonic stem cell-derived dopaminergic progenitors will differentiate to nigral dopaminergic neurons, integrate into appropriate neuronal circuits, and regrow axons to innervate their natural targets (Cardoso et al., 2018; Grealish et al., 2014). It, therefore, may be possible to obtain mice with human dopaminergic neurons, correctly differentiated and integrated into host neuronal circuits, essentially replacing the endogenous dopaminergic system. A similar strategy has already been successfully implemented to obtain humanized mice with brains chimeric for human glia (Windrem et al., 2014).
LITERATURE CITED
- Abdelmotilib, H., Maltbie, T., Delic, V., Liu, Z., Hu, X., Fraser, K. B., … West, A. (2017). α-Synuclein fibril-induced inclusion spread in rats and mice correlates with dopaminergic neurodegeneration. Neurobiology of Disease , 105, 84–98. doi: 10.1016/j.nbd.2017.05.014.
- Airavaara, M., Harvey, B. K., Voutilainen, M. H., Shen, H., Chou, J., Lindholm, P., … Wang, Y. (2012). CDNF protects the nigrostriatal dopamine system and promotes recovery after MPTP treatment in mice. Cell Transplantation , 21(6), 1213–1223. doi: 10.3727/096368911X600948.
- Airavaara, M., Voutilainen, M. H., Wang, Y., & Hoffer, B. (2012). Neurorestoration. Parkinsonism & Related Disorders, 18(Suppl 1), S143–S146. doi: 10.1016/S1353-8020(11)70045-1.
- Akundi, R. S., Huang, Z., Eason, J., Pandya, J. D., Zhi, L., Cass, W. A., … Bueler, H. (2011). Increased mitochondrial calcium sensitivity and abnormal expression of innate immunity genes precede dopaminergic defects in Pink1-deficient mice. PLoS One , 6(1), e16038. doi: 10.1371/journal.pone.0016038.
- Alam, M., & Schmidt, W. J. (2002). Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behavioural Brain Research , 136(1), 317–324. doi: 10.1016/s0166-4328(02)00180-8.
- Albert, K., Voutilainen, M. H., Domanskyi, A., & Airavaara, M. (2017). AAV vector-mediated gene delivery to substantia nigra dopamine neurons: Implications for gene therapy and disease models. Genes (Basel) , 8(2), E63. doi: 10.3390/genes8020063.
- Albert, K., Voutilainen, M. H., Domanskyi, A., Piepponen, T. P., Ahola, S., Tuominen, R. K., … Airavaara, M. (2019). Downregulation of tyrosine hydroxylase phenotype after AAV injection above substantia nigra: Caution in experimental models of Parkinson's disease. Journal of Neuroscience Research , 97(3), 346–361. doi: 10.1002/jnr.24363.
- Andres-Mateos, E., Perier, C., Zhang, L., Blanchard-Fillion, B., Greco, T. M., Thomas, B., … Dawson, V. L. (2007). DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proceedings of the National Academy of Sciences of the United States of America , 104(37), 14807–14812. doi: 10.1073/pnas.0703219104.
- Asakawa, T., Fang, H., Sugiyama, K., Nozaki, T., Hong, Z., Yang, Y., … Xia, Y. (2016). Animal behavioral assessments in current research of Parkinson's disease. Neuroscience and Biobehavioral Reviews , 65, 63–94. doi: 10.1016/j.neubiorev.2016.03.016.
- Back, S., Raki, M., Tuominen, R. K., Raasmaja, A., Bergstrom, K., & Mannisto, P. T. (2013). High correlation between in vivo [123I]β-CIT SPECT/CT imaging and post-mortem immunohistochemical findings in the evaluation of lesions induced by 6-OHDA in rats. EJNMMI Research , 3(1), 46. doi: 10.1186/2191-219X-3-46.
- Backman, C. M., Malik, N., Zhang, Y., Shan, L., Grinberg, A., Hoffer, B. J., … Tomac, A. C. (2006). Characterization of a mouse strain expressing Cre recombinase from the 3′ untranslated region of the dopamine transporter locus. Genesis , 44(8), 383–390. doi: 10.1002/dvg.20228.
- Bartels, A. L., & Leenders, K. L. (2009). Parkinson's disease: The syndrome, the pathogenesis and pathophysiology. Cortex , 45(8), 915–921. doi: 10.1016/j.cortex.2008.11.010.
- Bastias-Candia, S., Di Benedetto, M., D'Addario, C., Candeletti, S., & Romualdi, P. (2015). Combined exposure to agriculture pesticides, paraquat and maneb, induces alterations in the N/OFQ-NOPr and PDYN/KOPr systems in rats: Relevance to sporadic Parkinson's disease. Environmental Toxicology , 30(6), 656–663. doi: 10.1002/tox.21943.
- Beevers, J. E., Caffrey, T. M., & Wade-Martins, R. (2013). Induced pluripotent stem cell (iPSC)-derived dopaminergic models of Parkinson's disease. Biochemical Society Transactions , 41(6), 1503–1508. doi: 10.1042/BST20130194.
- Bengoa-Vergniory, N., Roberts, R. F., Wade-Martins, R., & Alegre-Abarrategui, J. (2017). Alpha-synuclein oligomers: A new hope. Acta Neuropathologica , 134(6), 819–838. doi: 10.1007/s00401-017-1755-1.
- Bentea, E., Verbruggen, L., & Massie, A. (2017). The proteasome inhibition model of Parkinson's disease. Journal of Parkinson's Disease , 7(1), 31–63. doi: 10.3233/JPD-160921.
- Bernheimer, H., Birkmayer, W., Hornykiewicz, O., Jellinger, K., & Seitelberger, F. (1973). Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. Journal of the Neurological Sciences , 20(4), 415–455. doi: 10.1016/0022-510X(73)90175-5
- Betarbet, R., Sherer, T. B., MacKenzie, G., Garcia-Osuna, M., Panov, A. V., & Greenamyre, J. T. (2000). Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nature Neuroscience , 3(12), 1301–1306. doi: 10.1038/81834.
- Bichler, Z., Lim, H. C., Zeng, L., & Tan, E. K. (2013). Non-motor and motor features in LRRK2 transgenic mice. PLoS One , 8(7), e70249. doi: 10.1371/journal.pone.0070249.
- Blumenstock, S., Rodrigues, E. F., Peters, F., Blazquez-Llorca, L., Schmidt, F., Giese, A., & Herms, J. (2017). Seeding and transgenic overexpression of alpha-synuclein triggers dendritic spine pathology in the neocortex. EMBO Molecular Medicine , 9(5), 716–731. doi: 10.15252/emmm.201607305.
- Bonito-Oliva, A., Masini, D., & Fisone, G. (2014). A mouse model of non-motor symptoms in Parkinson's disease: Focus on pharmacological interventions targeting affective dysfunctions. Frontiers in Behavioral Neuroscience , 8, 290. doi: 10.3389/fnbeh.2014.00290.
- Bousset, L., Pieri, L., Ruiz-Arlandis, G., Gath, J., Jensen, P. H., Habenstein, B., … Melki, R. (2013). Structural and functional characterization of two alpha-synuclein strains. Nature Communications , 4, 2575. doi: 10.1038/ncomms3575.
- Boutros, M., Heigwer, F., & Laufer, C. (2015). Microscopy-based high-content screening. Cell , 163(6), 1314–1325. doi: 10.1016/j.cell.2015.11.007.
- Braak, H., Del Tredici, K., Rub, U., de Vos, R. A., Jansen Steur, E. N., & Braak, E. (2003). Staging of brain pathology related to sporadic Parkinson's disease. Neurobiology of Aging , 24(2), 197–211. doi: 10.1016/s0197-4580(02)00065-9.
- Breger, L. S., & Fuzzati Armentero, M. T. (2019). Genetically engineered animal models of Parkinson's disease: From worm to rodent. European Journal of Neuroscience , 49(4), 533–560. doi: 10.1111/ejn.14300.
- Briggs, C. E., Wang, Y., Kong, B., Woo, T. U., Iyer, L. K., & Sonntag, K. C. (2015). Midbrain dopamine neurons in Parkinson's disease exhibit a dysregulated miRNA and target-gene network. Brain Research , 1618, 111–121. doi: 10.1016/j.brainres.2015.05.021.
- Burbulla, L. F., Song, P., Mazzulli, J. R., Zampese, E., Wong, Y. C., Jeon, S., … Krainc, D. (2017). Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson's disease. Science , 357(6357), 1255–1261. doi: 10.1126/science.aam9080.
- Burke, R. E., Cadet, J. L., Kent, J. D., Karanas, A. L., & Jackson-Lewis, V. (1990). An assessment of the validity of densitometric measures of striatal tyrosine hydroxylase-positive fibers: Relationship to apomorphine-induced rotations in 6-hydroxydopamine lesioned rats. Journal of Neuroscience Methods , 35(1), 63–73. doi: 10.1016/0165-0270(90)90095-w.
- Burke, R. E., Dauer, W. T., & Vonsattel, J. P. (2008). A critical evaluation of the Braak staging scheme for Parkinson's disease. Annals of Neurology , 64(5), 485–491. doi: 10.1002/ana.21541.
- Cannon, J. R., Tapias, V., Na, H. M., Honick, A. S., Drolet, R. E., & Greenamyre, J. T. (2009). A highly reproducible rotenone model of Parkinson's disease. Neurobiology of Disease , 34(2), 279–290. doi: 10.1016/j.nbd.2009.01.016.
- Caputi, F. F., Carretta, D., Lattanzio, F., Palmisano, M., Candeletti, S., & Romualdi, P. (2015). Proteasome subunit and opioid receptor gene expression down-regulation induced by paraquat and maneb in human neuroblastoma SH-SY5Y cells. Environmental Toxicology and Pharmacology , 40(3), 895–900. doi: 10.1016/j.etap.2015.09.019.
- Cardoso, T., Adler, A. F., Mattsson, B., Hoban, D. B., Nolbrant, S., Wahlestedt, J. N., … Parmar, M. (2018). Target-specific forebrain projections and appropriate synaptic inputs of hESC-derived dopamine neurons grafted to the midbrain of parkinsonian rats. Journal of Comparative Neurology , 526(13), 2133–2146. doi: 10.1002/cne.24500.
- Carlsson, A. (2001). A paradigm shift in brain research. Science , 294(5544), 1021–1024. doi: 10.1126/science.1066969.
- Carlsson, A., Lindqvist, M., & Magnusson, T. (1957). 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature , 180(4596), 1200. doi: 10.1038/1801200a0.
- Caudle, W. M., Richardson, J. R., Wang, M. Z., Taylor, T. N., Guillot, T. S., McCormack, A. L., … Miller, G. W. (2007). Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. Journal of Neuroscience , 27(30), 8138–8148. doi: 10.1523/JNEUROSCI.0319-07.2007.
- Chambers, S. M., Fasano, C. A., Papapetrou, E. P., Tomishima, M., Sadelain, M., & Studer, L. (2009). Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nature Biotechnology , 27(3), 275–280. doi: 10.1038/nbt.1529.
- Chan, C. S., Guzman, J. N., Ilijic, E., Mercer, J. N., Rick, C., Tkatch, T., … Surmeier, D. J. (2007). ‘Rejuvenation’ protects neurons in mouse models of Parkinson's disease. Nature , 447(7148), 1081–1086. doi: 10.1038/nature05865.
- Chandran, J. S., Lin, X., Zapata, A., Hoke, A., Shimoji, M., Moore, S. O., … Cai, H. (2008). Progressive behavioral deficits in DJ-1-deficient mice are associated with normal nigrostriatal function. Neurobiology of Disease , 29(3), 505–514. doi: 10.1016/j.nbd.2007.11.011.
- Chen, C., Li, X., Ge, G., Liu, J., Biju, K. C., Laing, S. D., … Li, S. (2018). GDNF-expressing macrophages mitigate loss of dopamine neurons and improve Parkinsonian symptoms in MitoPark mice. Scientific Reports , 8(1), 5460. doi: 10.1038/s41598-018-23795-4.
- Chen, C. Y., Weng, Y. H., Chien, K. Y., Lin, K. J., Yeh, T. H., Cheng, Y. P., … Wang, H. L. (2012). (G2019S) LRRK2 activates MKK4-JNK pathway and causes degeneration of SN dopaminergic neurons in a transgenic mouse model of PD. Cell Death and Differentiation , 19(10), 1623–1633. doi: 10.1038/cdd.2012.42.
- Chen, L., Cagniard, B., Mathews, T., Jones, S., Koh, H. C., Ding, Y., … Zhuang, X. (2005). Age-dependent motor deficits and dopaminergic dysfunction in DJ-1 null mice. Journal of Biological Chemistry , 280(22), 21418–21426. doi: 10.1074/jbc.M413955200.
- Chen, Y. H., Harvey, B. K., Hoffman, A. F., Wang, Y., Chiang, Y. H., & Lupica, C. R. (2008). MPTP-induced deficits in striatal synaptic plasticity are prevented by glial cell line-derived neurotrophic factor expressed via an adeno-associated viral vector. FASEB Journal , 22(1), 261–275. doi: 10.1096/fj.07-8797com.
- Cheng, H. C., Kim, S. R., Oo, T. F., Kareva, T., Yarygina, O., Rzhetskaya, M., … Burke, R. E. (2011). Akt suppresses retrograde degeneration of dopaminergic axons by inhibition of macroautophagy. Journal of Neuroscience , 31(6), 2125–2135. doi: 10.1523/JNEUROSCI.5519-10.2011.
- Cheng, H. C., Ulane, C. M., & Burke, R. E. (2010). Clinical progression in Parkinson disease and the neurobiology of axons. Annals of Neurology , 67(6), 715–725. doi: 10.1002/ana.21995.
- Chmielarz, P., Er, Ş., Konovalova, J., Bandrés, L., Hlushchuk, I., Albert, K., … Domanskyi, A. (2019). GDNF/RET signaling pathway activation eliminates Lewy Body pathology in midbrain dopamine neurons. bioRxiv , 752899. doi: 10.1101/752899.
- Chmielarz, P., Konovalova, J., Najam, S. S., Alter, H., Piepponen, T. P., Erfle, H., … Domanskyi, A. (2017). Dicer and microRNAs protect adult dopamine neurons. Cell Death & Disease, 8(5), e2813. doi: 10.1038/cddis.2017.214.
- Chu, Y., Muller, S., Tavares, A., Barret, O., Alagille, D., Seibyl, J., … Kordower, J. H. (2019). Intrastriatal alpha-synuclein fibrils in monkeys: Spreading, imaging and neuropathological changes. Brain , 142(11), 3565–3579. doi: 10.1093/brain/awz296.
- Cicchetti, F., Brownell, A. L., Williams, K., Chen, Y. I., Livni, E., & Isacson, O. (2002). Neuroinflammation of the nigrostriatal pathway during progressive 6-OHDA dopamine degeneration in rats monitored by immunohistochemistry and PET imaging. European Journal of Neuroscience , 15(6), 991–998. doi: 10.1046/j.1460-9568.2002.01938.x.
- Clark, E. P. (1929). The occurrence of rotenone in the Peruvian fish poison “Cube”. Science , 70(1820), 478–479. doi: 10.1126/science.70.1820.478-a.
- Corasaniti, M. T., Defilippo, R., Rodino, P., Nappi, G., & Nistico, G. (1991). Evidence that paraquat is able to cross the blood-brain barrier to a different extent in rats of various age. Functional Neurology , 6(4), 385–391.
- Day, B. J., Patel, M., Calavetta, L., Chang, L. Y., & Stamler, J. S. (1999). A mechanism of paraquat toxicity involving nitric oxide synthase. Proceedings of the National Academy of Sciences of the United States of America , 96(22), 12760–12765. doi: 10.1073/pnas.96.22.12760.
- Decressac, M., Mattsson, B., & Bjorklund, A. (2012). Comparison of the behavioural and histological characteristics of the 6-OHDA and alpha-synuclein rat models of Parkinson's disease. Experimental Neurology , 235(1), 306–315. doi: 10.1016/j.expneurol.2012.02.012.
- Decressac, M., Mattsson, B., Lundblad, M., Weikop, P., & Bjorklund, A. (2012). Progressive neurodegenerative and behavioural changes induced by AAV-mediated overexpression of alpha-synuclein in midbrain dopamine neurons. Neurobiology of Disease , 45(3), 939–953. doi: 10.1016/j.nbd.2011.12.013.
- Decressac, M., Mattsson, B., Weikop, P., Lundblad, M., Jakobsson, J., & Bjorklund, A. (2013). TFEB-mediated autophagy rescues midbrain dopamine neurons from alpha-synuclein toxicity. Proceedings of the National Academy of Sciences of the United States of America , 110(19), E1817–E1826. doi: 10.1073/pnas.1305623110.
- Decressac, M., Ulusoy, A., Mattsson, B., Georgievska, B., Romero-Ramos, M., Kirik, D., & Bjorklund, A. (2011). GDNF fails to exert neuroprotection in a rat alpha-synuclein model of Parkinson's disease. Brain , 134(Pt 8), 2302–2311. doi: 10.1093/brain/awr149.
- Delenclos, M., Burgess, J. D., Lamprokostopoulou, A., Outeiro, T. F., Vekrellis, K., & McLean, P. J. (2019). Cellular models of alpha-synuclein toxicity and aggregation. Journal of Neurochemistry , 150(5), 566–576. doi: 10.1111/jnc.14806.
- Deneyer, L., Albertini, G., Bentea, E., & Massie, A. (2019). Systemic LPS-induced neuroinflammation increases the susceptibility for proteasome inhibition-induced degeneration of the nigrostriatal pathway. Parkinsonism & Related Disorders, 68, 26–32. doi: 10.1016/j.parkreldis.2019.09.025.
- Domanskyi, A., Alter, H., Vogt, M. A., Gass, P., & Vinnikov, I. A. (2014). Transcription factors Foxa1 and Foxa2 are required for adult dopamine neurons maintenance. Frontiers in Cellular Neuroscience , 8, 275. doi: 10.3389/fncel.2014.00275.
- Domanskyi, A., Geissler, C., Vinnikov, I. A., Alter, H., Schober, A., Vogt, M. A., … Schutz, G. (2011). Pten ablation in adult dopaminergic neurons is neuroprotective in Parkinson's disease models. FASEB Journal , 25(9), 2898–2910. doi: 10.1096/fj.11-181958.
- Domanskyi, A., Saarma, M., & Airavaara, M. (2015). Prospects of neurotrophic factors for parkinson's disease: comparison of protein and gene therapy. Hum Gene therapy , 26(8), 550–559. doi:10.1089/hum.2015.065.
- Eisner, D. A. (2018). Reproducibility of science: Fraud, impact factors and carelessness. Journal of Molecular and Cellular Cardiology , 114, 364–368. doi: 10.1016/j.yjmcc.2017.10.009.
- Ekstrand, M. I., & Galter, D. (2009). The MitoPark Mouse–an animal model of Parkinson's disease with impaired respiratory chain function in dopamine neurons. Parkinsonism & Related Disorders, 15(Suppl 3), S185–S188. doi: 10.1016/S1353-8020(09)70811-9.
- Ekstrand, M. I., Terzioglu, M., Galter, D., Zhu, S., Hofstetter, C., Lindqvist, E., … Larsson, N. G. (2007). Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proceedings of the National Academy of Sciences of the United States of America , 104(4), 1325–1330. doi: 10.1073/pnas.0605208103.
- Engblom, D., Bilbao, A., Sanchis-Segura, C., Dahan, L., Perreau-Lenz, S., Balland, B., … Spanagel, R. (2008). Glutamate receptors on dopamine neurons control the persistence of cocaine seeking. Neuron , 59(3), 497–508. doi: 10.1016/j.neuron.2008.07.010.
- Espa, E., Clemensson, E. K. H., Luk, K. C., Heuer, A., Bjorklund, T., & Cenci, M. A. (2019). Seeding of protein aggregation causes cognitive impairment in rat model of cortical synucleinopathy. Movement Disorders , 34(11), 1699–1710. doi: 10.1002/mds.27810.
- Evsyukov, V., Domanskyi, A., Bierhoff, H., Gispert, S., Mustafa, R., Schlaudraff, F., … Parlato, R. (2017). Genetic mutations linked to Parkinson's disease differentially control nucleolar activity in pre-symptomatic mouse models. Disease Models & Mechanisms, 10(5), 633–643. doi: 10.1242/dmm.028092.
- Falkenburger, B. H., Saridaki, T., & Dinter, E. (2016). Cellular models for Parkinson's disease. Journal of Neurochemistry , 139(Suppl 1), 121–130. doi: 10.1111/jnc.13618.
- Falkenburger, B. H., & Schulz, J. B. (2006). Limitations of cellular models in Parkinson's disease research. Journal of Neural Transmission Supplementum , (70), 261–268. doi: 10.1007/978-3-211-45295-0_40.
- Faull, R. L., & Laverty, R. (1969). Changes in dopamine levels in the corpus striatum following lesions in the substantia nigra. Experimental Neurology , 23(3), 332–340. doi: 10.1016/0014-4886(69)90081-8.
- Febbraro, F., Sahin, G., Farran, A., Soares, S., Jensen, P. H., Kirik, D., & Romero-Ramos, M. (2013). Ser129D mutant alpha-synuclein induces earlier motor dysfunction while S129A results in distinctive pathology in a rat model of Parkinson's disease. Neurobiology of Disease , 56, 47–58. doi: 10.1016/j.nbd.2013.03.014.
- Fei, Q., McCormack, A. L., Di Monte, D. A., & Ethell, D. W. (2008). Paraquat neurotoxicity is mediated by a Bak-dependent mechanism. Journal of Biological Chemistry , 283(6), 3357–3364. doi: 10.1074/jbc.M708451200.
- Ferrante, R. J., Schulz, J. B., Kowall, N. W., & Beal, M. F. (1997). Systemic administration of rotenone produces selective damage in the striatum and globus pallidus, but not in the substantia nigra. Brain Research , 753(1), 157–162. doi: 10.1016/s0006-8993(97)00008-5.
- Fornai, F., Schluter, O. M., Lenzi, P., Gesi, M., Ruffoli, R., Ferrucci, M., … Sudhof, T. C. (2005). Parkinson-like syndrome induced by continuous MPTP infusion: Convergent roles of the ubiquitin-proteasome system and alpha-synuclein. Proceedings of the National Academy of Sciences of the United States of America , 102(9), 3413–3418. doi: 10.1073/pnas.0409713102.
- Galter, D., Pernold, K., Yoshitake, T., Lindqvist, E., Hoffer, B., Kehr, J., … Olson, L. (2010). MitoPark mice mirror the slow progression of key symptoms and L-DOPA response in Parkinson's disease. Genes, Brain, and Behavior , 9(2), 173–181. doi: 10.1111/j.1601-183X.2009.00542.x.
- Gasser, T., Hardy, J., & Mizuno, Y. (2011). Milestones in PD genetics. Movement Disorders , 26(6), 1042–1048. doi: 10.1002/mds.23637.
- Ghaisas, S., Langley, M. R., Palanisamy, B. N., Dutta, S., Narayanaswamy, K., Plummer, P. J., … Kanthasamy, A. G. (2019). MitoPark transgenic mouse model recapitulates the gastrointestinal dysfunction and gut-microbiome changes of Parkinson's disease. Neurotoxicology , 75, 186–199. doi: 10.1016/j.neuro.2019.09.004.
- Ghosh, A., Tyson, T., George, S., Hildebrandt, E. N., Steiner, J. A., Madaj, Z., … Brundin, P. (2016). Mitochondrial pyruvate carrier regulates autophagy, inflammation, and neurodegeneration in experimental models of Parkinson's disease. Science Translational Medicine , 8(368), 368ra174. doi: 10.1126/scitranslmed.aag2210.
- Gispert, S., Ricciardi, F., Kurz, A., Azizov, M., Hoepken, H. H., Becker, D., … Auburger, G.. (2009). Parkinson phenotype in aged PINK1-deficient mice is accompanied by progressive mitochondrial dysfunction in absence of neurodegeneration. PLoS One , 4(6), e5777. doi: 10.1371/journal.pone.0005777.
- Glasl, L., Kloos, K., Giesert, F., Roethig, A., Di Benedetto, Kuhn, B. R., … Wurst, W. (2012). Pink1-deficiency in mice impairs gait, olfaction and serotonergic innervation of the olfactory bulb. Experimental Neurology , 235(1), 214–227. doi: 10.1016/j.expneurol.2012.01.002.
- Glinka, Y., Gassen, M., & Youdim, M. B. (1997). Mechanism of 6-hydroxydopamine neurotoxicity. Journal of Neural Transmission Supplementum , 50, 55–66. doi: 10.1007/978-3-7091-6842-4_7.
- Goldberg, M. S., Fleming, S. M., Palacino, J. J., Cepeda, C., Lam, H. A., Bhatnagar, A., … Shen, J. (2003). Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. Journal of Biological Chemistry , 278(44), 43628–43635. doi: 10.1074/jbc.M308947200.
- Goldberg, M. S., Pisani, A., Haburcak, M., Vortherms, T. A., Kitada, T., Costa, C., … Shen, J. (2005). Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron , 45(4), 489–496. doi: 10.1016/j.neuron.2005.01.041.
- Gombash, S. E., Manfredsson, F. P., Kemp, C. J., Kuhn, N. C., Fleming, S. M., Egan, A. E., … Sortwell, C. E. (2013). Morphological and behavioral impact of AAV2/5-mediated overexpression of human wildtype alpha-synuclein in the rat nigrostriatal system. PLoS One , 8(11), e81426. doi: 10.1371/journal.pone.0081426.
- Gonzalez-Reyes, L. E., Verbitsky, M., Blesa, J., Jackson-Lewis, V., Paredes, D., Tillack, K., … Kottmann, A. H. (2012). Sonic hedgehog maintains cellular and neurochemical homeostasis in the adult nigrostriatal circuit. Neuron , 75(2), 306–319. doi: 10.1016/j.neuron.2012.05.018.
- Graham, D. G. (1978). Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Molecular Pharmacology , 14(4), 633–643.
- Grealish, S., Diguet, E., Kirkeby, A., Mattsson, B., Heuer, A., Bramoulle, Y., … Parmar, M. (2014). Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson's disease. Cell Stem Cell , 15(5), 653–665. doi: 10.1016/j.stem.2014.09.017.
- Greenamyre, J. T., Cannon, J. R., Drolet, R., & Mastroberardino, P. G. (2010). Lessons from the rotenone model of Parkinson's disease. Trends in Pharmacological Sciences , 31(4), 141–142; author reply 142-143. doi: 10.1016/j.tips.2009.12.006.
- Gribaudo, S., Tixador, P., Bousset, L., Fenyi, A., Lino, P., Melki, R., … Perrier, A. L. (2019). Propagation of alpha-synuclein strains within human reconstructed neuronal network. Stem Cell Reports , 12(2), 230–244. doi: 10.1016/j.stemcr.2018.12.007.
- Guzman, J. N., Sanchez-Padilla, J., Wokosin, D., Kondapalli, J., Ilijic, E., Schumacker, P. T., & Surmeier, D. J. (2010). Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature , 468(7324), 696–700. doi: 10.1038/nature09536.
- Hagg, T., Manthorpe, M., Vahlsing, H. L., & Varon, S. (1988). Delayed treatment with nerve growth factor reverses the apparent loss of cholinergic neurons after acute brain damage. Experimental Neurology , 101(2), 303–312. doi: 10.1016/0014-4886(88)90013-1.
- Halliday, G., Lees, A., & Stern, M. (2011). Milestones in Parkinson's disease–clinical and pathologic features. Movement Disorders , 26(6), 1015–1021. doi: 10.1002/mds.23669.
- Hanell, A., & Marklund, N. (2014). Structured evaluation of rodent behavioral tests used in drug discovery research. Frontiers in Behavioral Neuroscience , 8, 252. doi: 10.3389/fnbeh.2014.00252.
- Hefti, F., Melamed, E., & Wurtman, R. J. (1980). Partial lesions of the dopaminergic nigrostriatal system in rat brain: Biochemical characterization. Brain Research , 195(1), 123–137. doi: 10.1016/0006-8993(80)90871-9.
- Heikkila, R. E., Nicklas, W. J., Vyas, I., & Duvoisin, R. C. (1985). Dopaminergic toxicity of rotenone and the 1-methyl-4-phenylpyridinium ion after their stereotaxic administration to rats: Implication for the mechanism of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity. Neuroscience Letters , 62(3), 389–394. doi: 10.1016/0304-3940(85)90580-4.
- Herzig, M. C., Bidinosti, M., Schweizer, T., Hafner, T., Stemmelen, C., Weiss, A., … Shimshek, D. R. (2012). High LRRK2 levels fail to induce or exacerbate neuronal alpha-synucleinopathy in mouse brain. PLoS One , 7(5), e36581. doi: 10.1371/journal.pone.0036581.
- Heuer, A., Smith, G. A., Lelos, M. J., Lane, E. L., & Dunnett, S. B. (2012). Unilateral nigrostriatal 6-hydroxydopamine lesions in mice I: Motor impairments identify extent of dopamine depletion at three different lesion sites. Behavioural Brain Research , 228(1), 30–43. doi: 10.1016/j.bbr.2011.11.027.
- Hirsch, E. C., & Hunot, S. (2009). Neuroinflammation in Parkinson's disease: A target for neuroprotection? Lancet Neurology , 8(4), 382–397. doi: 10.1016/S1474-4422(09)70062-6.
- Hoglinger, G. U., Feger, J., Prigent, A., Michel, P. P., Parain, K., Champy, P., … Hirsch, E. C. (2003). Chronic systemic complex I inhibition induces a hypokinetic multisystem degeneration in rats. Journal of Neurochemistry , 84(3), 491–502. doi: 10.1046/j.1471-4159.2003.01533.x.
- Hudson, J. L., Fong, C. S., Boyson, S. J., & Hoffer, B. J. (1994). Conditioned apomorphine-induced turning in 6-OHDA-lesioned rats. Pharmacology, Biochemistry and Behavior , 49(1), 147–154. doi: 10.1016/0091-3057(94)90469-3.
- Hudson, J. L., van Horne, C. G., Stromberg, I., Brock, S., Clayton, J., Masserano, J., … Gerhardt, G. A. (1993). Correlation of apomorphine- and amphetamine-induced turning with nigrostriatal dopamine content in unilateral 6-hydroxydopamine lesioned rats. Brain Research , 626(1-2), 167–174. doi: 10.1016/0006-8993(93)90576-9.
- Huotarinen, A., Penttinen, A. M., Back, S., Voutilainen, M. H., Julku, U., Piepponen, T. P., … Airavaara, M. (2018). Combination of CDNF and deep brain stimulation decreases neurological deficits in late-stage model Parkinson's disease. Neuroscience , 374, 250–263. doi: 10.1016/j.neuroscience.2018.01.052.
- Itier, J. M., Ibanez, P., Mena, M. A., Abbas, N., Cohen-Salmon, C., Bohme, G. A., … Garcia de Yebenes, J. (2003). Parkin gene inactivation alters behaviour and dopamine neurotransmission in the mouse. Human Molecular Genetics , 12(18), 2277–2291. doi: 10.1093/hmg/ddg239.
- Jackson-Lewis, V., & Przedborski, S. (2007). Protocol for the MPTP mouse model of Parkinson's disease. Nature Protocols , 2(1), 141–151. doi: 10.1038/nprot.2006.342.
- Jonsson, G., Sundstrom, E., Mefford, I., Olson, L., Johnson, S., Freedman, R., & Hoffer, B. (1985). Electrophysiological and neurochemical correlates of the neurotoxic effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on central catecholamine neurons in the mouse. Naunyn-Schmiedebergs Archives of Pharmacology , 331(1), 1–6. doi: 10.1007/bf00498844.
- Kadkhodaei, B., Alvarsson, A., Schintu, N., Ramskold, D., Volakakis, N., Joodmardi, E., … Perlmann, T. (2013). Transcription factor Nurr1 maintains fiber integrity and nuclear-encoded mitochondrial gene expression in dopamine neurons. Proceedings of the National Academy of Sciences of the United States of America , 110(6), 2360–2365. doi: 10.1073/pnas.1221077110.
- Karampetsou, M., Ardah, M. T., Semitekolou, M., Polissidis, A., Samiotaki, M., Kalomoiri, M., … Vekrellis, K. (2017). Phosphorylated exogenous alpha-synuclein fibrils exacerbate pathology and induce neuronal dysfunction in mice. Scientific Reports , 7(1), 16533. doi: 10.1038/s41598-017-15813-8.
- Kepp, O., Galluzzi, L., Lipinski, M., Yuan, J., & Kroemer, G. (2011). Cell death assays for drug discovery. Nature Reviews Drug Discovery , 10(3), 221–237. doi: 10.1038/nrd3373.
- Kim, H. W., Choi, W. S., Sorscher, N., Park, H. J., Tronche, F., Palmiter, R. D., & Xia, Z. (2015). Genetic reduction of mitochondrial complex I function does not lead to loss of dopamine neurons in vivo. Neurobiology of Aging , 36(9), 2617–2627. doi: 10.1016/j.neurobiolaging.2015.05.008.
- Kim, J., Inoue, K., Ishii, J., Vanti, W. B., Voronov, S. V., Murchison, E., … Abeliovich, A. (2007). A microRNA feedback circuit in midbrain dopamine neurons. Science , 317(5842), 1220–1224. doi: 10.1126/science.1140481.
- Kim, R. H., Smith, P. D., Aleyasin, H., Hayley, S., Mount, M. P., Pownall, S., … Mak, T. W. (2005). Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proceedings of the National Academy of Sciences of the United States of America , 102(14), 5215–5220. doi: 10.1073/pnas.0501282102.
- Kingsbury, A. E., Daniel, S. E., Sangha, H., Eisen, S., Lees, A. J., & Foster, O. J. (2004). Alteration in alpha-synuclein mRNA expression in Parkinson's disease. Movement Disorders , 19(2), 162–170. doi: 10.1002/mds.10683.
- Kirik, D., Rosenblad, C., & Bjorklund, A. (1998). Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Experimental Neurology , 152(2), 259–277. doi: 10.1006/exnr.1998.6848.
- Kish, S. J., Shannak, K., & Hornykiewicz, O. (1988). Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. New England Journal of Medicine , 318(14), 876–880. doi: 10.1056/NEJM198804073181402.
- Kitada, T., Asakawa, S., Hattori, N., Matsumine, H., Yamamura, Y., Minoshima, S., … Shimizu, N. (1998). Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature , 392(6676), 605–608. doi: 10.1038/33416.
- Kitada, T., Pisani, A., Porter, D. R., Yamaguchi, H., Tscherter, A., Martella, G., … Shen, J. (2007). Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proceedings of the National Academy of Sciences of the United States of America , 104(27), 11441–11446. doi: 10.1073/pnas.0702717104.
- Kitada, T., Tong, Y., Gautier, C. A., & Shen, J. (2009). Absence of nigral degeneration in aged parkin/DJ-1/PINK1 triple knockout mice. Journal of Neurochemistry , 111(3), 696–702. doi: 10.1111/j.1471-4159.2009.06350.x.
- Kittappa, R., Chang, W. W., Awatramani, R. B., & McKay, R. D. (2007). The foxa2 gene controls the birth and spontaneous degeneration of dopamine neurons in old age. PLoS Biology , 5(12), e325. doi: 10.1371/journal.pbio.0050325.
- Klein, C., & Westenberger, A. (2012). Genetics of Parkinson's disease. Cold Spring Harbor Perspectives in Medicine , 2(1), a008888. doi: 10.1101/cshperspect.a008888.
- Klein, R. L., Dayton, R. D., Leidenheimer, N. J., Jansen, K., Golde, T. E., & Zweig, R. M. (2006). Efficient neuronal gene transfer with AAV8 leads to neurotoxic levels of tau or green fluorescent proteins. Molecular Therapy , 13(3), 517–527. doi: 10.1016/j.ymthe.2005.10.008.
- Koprich, J. B., Johnston, T. H., Huot, P., Reyes, M. G., Espinosa, M., & Brotchie, J. M. (2011). Progressive neurodegeneration or endogenous compensation in an animal model of Parkinson's disease produced by decreasing doses of alpha-synuclein. PLoS One , 6(3), e17698. doi: 10.1371/journal.pone.0017698.
- Koprich, J. B., Kalia, L. V., & Brotchie, J. M. (2017). Animal models of alpha-synucleinopathy for Parkinson disease drug development. Nature Reviews Neuroscience , 18(9), 515–529. doi: 10.1038/nrn.2017.75.
- Kordower, J. H., Chu, Y., Hauser, R. A., Freeman, T. B., & Olanow, C. W. (2008). Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nature Medicine , 14(5), 504–506. doi: 10.1038/nm1747.
- Kordower, J. H., Kanaan, N. M., Chu, Y., Babu Suresh, Stansell, 3rd., R. J., Terpstra, B. T., … Collier, T. J. (2006). Failure of proteasome inhibitor administration to provide a model of Parkinson's disease in rats and monkeys. Annals of Neurology , 60(2), 264–268. doi: 10.1002/ana.20935.
- Kraus, O. Z., & Frey, B. J. (2016). Computer vision for high content screening. Critical Reviews in Biochemistry and Molecular Biology , 51(2), 102–109. doi: 10.3109/10409238.2015.1135868.
- Kreiner, G., Rafa-Zablocka, K., Barut, J., Chmielarz, P., Kot, M., Baginska, M., … Nalepa, I. (2019). Stimulation of noradrenergic transmission by reboxetine is beneficial for a mouse model of progressive parkinsonism. Scientific Reports , 9(1), 5262. doi: 10.1038/s41598-019-41756-3.
- Kristianto, J., Johnson, M. G., Zastrow, R. K., Radcliff, A. B., & Blank, R. D. (2017). Spontaneous recombinase activity of Cre-ERT2 in vivo. Transgenic Research , 26(3), 411–417. doi: 10.1007/s11248-017-0018-1.
- Kumer, S. C., & Vrana, K. E. (1996). Intricate regulation of tyrosine hydroxylase activity and gene expression. Journal of Neurochemistry , 67(2), 443–462. doi: 10.1046/j.1471-4159.1996.67020443.x.
- La Manno, G., Gyllborg, D., Codeluppi, S., Nishimura, K., Salto, C., Zeisel, A., … Linnarsson, S. (2016). Molecular diversity of midbrain development in mouse, human, and stem cells. Cell , 167(2), 566–580.e19. doi: 10.1016/j.cell.2016.09.027.
- Landeck, N., Buck, K., & Kirik, D. (2016). Toxic effects of human and rodent variants of alpha-synuclein in vivo. European Journal of Neuroscience , 1–12. doi: 10.1111/ejn.13493.
- Langley, M., Ghosh, A., Charli, A., Sarkar, S., Ay, M., Luo, J., … Kanthasamy, A. G. (2017). Mito-apocynin prevents mitochondrial dysfunction, microglial activation, oxidative damage, and progressive neurodegeneration in MitoPark transgenic mice. Antioxid Redox Signaling , 27(14), 1048–1066. doi: 10.1089/ars.2016.6905.
- Lee, S., Sterky, F. H., Mourier, A., Terzioglu, M., Cullheim, S., Olson, L., & Larsson, N. G. (2012). Mitofusin 2 is necessary for striatal axonal projections of midbrain dopamine neurons. Human Molecular Genetics , 21(22), 4827–4835. doi: 10.1093/hmg/dds352.
- Leroy, E., Boyer, R., Auburger, G., Leube, B., Ulm, G., Mezey, E., … Polymeropoulos, M. H. (1998). The ubiquitin pathway in Parkinson's disease. Nature , 395(6701), 451–452. doi: 10.1038/26652.
- Li, H., Jiang, H., Zhang, B., & Feng, J. (2018). Modeling Parkinson's disease using patient-specific induced pluripotent stem cells. Journal of Parkinson's Disease , 8(4), 479–493. doi: 10.3233/JPD-181353.
- Li, J. Y., Englund, E., Holton, J. L., Soulet, D., Hagell, P., Lees, A. J., … Brundin, P. (2008). Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nature Medicine , 14(5), 501–503. doi: 10.1038/nm1746.
- Li, X., Patel, J. C., Wang, J., Avshalumov, M. V., Nicholson, C., Buxbaum, J. D., … Yue, Z. (2010). Enhanced striatal dopamine transmission and motor performance with LRRK2 overexpression in mice is eliminated by familial Parkinson's disease mutation G2019S. Journal of Neuroscience , 30(5), 1788–1797. doi: 10.1523/JNEUROSCI.5604-09.2010.
- Li, Y., Liu, W., Oo, T. F., Wang, L., Tang, Y., Jackson-Lewis, V., … Li, C. (2009). Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson's disease. Nature Neuroscience , 12(7), 826–828. doi: 10.1038/nn.2349.
- Lin, X., Parisiadou, L., Gu, X. L., Wang, L., Shim, H., Sun, L., … Cai, H. (2009). Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson's-disease-related mutant alpha-synuclein. Neuron , 64(6), 807–827. doi: 10.1016/j.neuron.2009.11.006.
- Liss, B., Haeckel, O., Wildmann, J., Miki, T., Seino, S., & Roeper, J. (2005). K-ATP channels promote the differential degeneration of dopaminergic midbrain neurons. Nature Neuroscience , 8(12), 1742–1751. doi: 10.1038/nn1570.
- Liu, B. H., Wang, X., Ma, Y. X., & Wang, S. (2004). CMV enhancer/human PDGF-beta promoter for neuron-specific transgene expression. Gene Therapy , 11(1), 52–60. doi: 10.1038/sj.gt.3302126.
- Lopes, F. M., Bristot, I. J., da Motta, L. L., Parsons, R. B., & Klamt, F. (2017). Mimicking Parkinson's disease in a dish: Merits and pitfalls of the most commonly used dopaminergic in vitro models. NeuroMolecular Medicine , 19(2-3), 241–255. doi: 10.1007/s12017-017-8454-x.
- Lu, X. H., Fleming, S. M., Meurers, B., Ackerson, L. C., Mortazavi, F., Lo, V., … Yang, X. W. (2009). Bacterial artificial chromosome transgenic mice expressing a truncated mutant parkin exhibit age-dependent hypokinetic motor deficits, dopaminergic neuron degeneration, and accumulation of proteinase K-resistant alpha-synuclein. Journal of Neuroscience , 29(7), 1962–1976. doi: 10.1523/JNEUROSCI.5351-08.2009.
- Luk, K. C., Kehm, V., Carroll, J., Zhang, B., O'Brien, P., Trojanowski, J. Q., & Lee, V. M. (2012). Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science , 338(6109), 949–953. doi: 10.1126/science.1227157.
- Luk, K. C., Kehm, V. M., Zhang, B., O'Brien, P., Trojanowski, J. Q., & Lee, V. M. (2012). Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. Journal of Experimental Medicine , 209(5), 975–986. doi: 10.1084/jem.20112457.
- Luk, K. C., Song, C., O'Brien, P., Stieber, A., Branch, J. R., Brunden, K. R., … Lee, V. M. (2009). Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proceedings of the National Academy of Sciences of the United States of America , 106(47), 20051–20056. doi: 10.1073/pnas.0908005106.
- Lundblad, M., Usiello, A., Carta, M., Hakansson, K., Fisone, G., & Cenci, M. A. (2005). Pharmacological validation of a mouse model of l-DOPA-induced dyskinesia. Experimental Neurology , 194(1), 66–75. doi: 10.1016/j.expneurol.2005.02.002.
- Luthman, J., Fredriksson, A., Sundstrom, E., Jonsson, G., & Archer, T. (1989). Selective lesion of central dopamine or noradrenaline neuron systems in the neonatal rat: Motor behavior and monoamine alterations at adult stage. Behavioural Brain Research , 33(3), 267–277. doi: 10.1016/s0166-4328(89)80121-4.
- Maekawa, T., Mori, S., Sasaki, Y., Miyajima, T., Azuma, S., Ohta, E., & Obata, F. (2012). The I2020T leucine-rich repeat kinase 2 transgenic mouse exhibits impaired locomotive ability accompanied by dopaminergic neuron abnormalities. Molecular Neurodegeneration , 7, 15. doi: 10.1186/1750-1326-7-15.
- Manfredsson, F. P., Luk, K. C., Benskey, M. J., Gezer, A., Garcia, J., Kuhn, N. C., … Kordower, J. H. (2018). Induction of alpha-synuclein pathology in the enteric nervous system of the rat and non-human primate results in gastrointestinal dysmotility and transient CNS pathology. Neurobiology of Disease , 112, 106–118. doi: 10.1016/j.nbd.2018.01.008.
- Manning-Bog, A. B., Reaney, S. H., Chou, V. P., Johnston, L. C., McCormack, A. L., Johnston, J., … Di Monte, D. A. (2006). Lack of nigrostriatal pathology in a rat model of proteasome inhibition. Annals of Neurology , 60(2), 256–260. doi: 10.1002/ana.20938.
- Mansour, A. A., Goncalves, J. T., Bloyd, C. W., Li, H., Fernandes, S., Quang, D., … Gage, F. H. (2018). An in vivo model of functional and vascularized human brain organoids. Nature Biotechnology , 36(5), 432–441. doi: 10.1038/nbt.4127.
- Marin, C., Rodriguez-Oroz, M. C., & Obeso, J. A. (2006). Motor complications in Parkinson's disease and the clinical significance of rotational behavior in the rat: Have we wasted our time? Experimental Neurology , 197(2), 269–274. doi: 10.1016/j.expneurol.2005.11.002.
- Matsuda, W., Furuta, T., Nakamura, K. C., Hioki, H., Fujiyama, F., Arai, R., & Kaneko, T. (2009). Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. Journal of Neuroscience , 29(2), 444–453. doi: 10.1523/JNEUROSCI.4029-08.2009.
- Mazzio, E. A., Reams, R. R., & Soliman, K. F. (2004). The role of oxidative stress, impaired glycolysis and mitochondrial respiratory redox failure in the cytotoxic effects of 6-hydroxydopamine in vitro. Brain Research , 1004(1–2), 29–44. doi: 10.1016/j.brainres.2003.12.034.
- McCormack, A. L., & Di Monte, D. A. (2003). Effects of L-dopa and other amino acids against paraquat-induced nigrostriatal degeneration. Journal of Neurochemistry , 85(1), 82–86. doi: 10.1046/j.1471-4159.2003.01621.x.
- McCormack, A. L., Thiruchelvam, M., Manning-Bog, A. B., Thiffault, C., Langston, J. W., Cory-Slechta, D. A., & Di Monte, D. A. (2002). Environmental risk factors and Parkinson's disease: Selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiology of Disease , 10(2), 119–127. doi: 10.1006/nbdi.2002.0507.
- McNaught, K. S., Belizaire, R., Jenner, P., Olanow, C. W., & Isacson, O. (2002). Selective loss of 20S proteasome alpha-subunits in the substantia nigra pars compacta in Parkinson's disease. Neuroscience Letters , 326(3), 155–158. doi: 10.1016/s0304-3940(02)00296-3.
- McNaught, K. S., Bjorklund, L. M., Belizaire, R., Isacson, O., Jenner, P., & Olanow, C. W. (2002). Proteasome inhibition causes nigral degeneration with inclusion bodies in rats. Neuroreport , 13(11), 1437–1441. doi: 10.1097/00001756-200208070-00018.
- McNaught, K. S., & Jenner, P. (2001). Proteasomal function is impaired in substantia nigra in Parkinson's disease. Neuroscience Letters , 297(3), 191–194. doi: 10.1016/s0304-3940(00)01701-8.
- McQuin, C., Goodman, A., Chernyshev, V., Kamentsky, L., Cimini, B. A., Karhohs, K. W., … Carpenter, A. E. (2018). CellProfiler 3.0: Next-generation image processing for biology. PLoS Biology , 16(7), e2005970. doi: 10.1371/journal.pbio.2005970.
- Meiser, J., Weindl, D., & Hiller, K. (2013). Complexity of dopamine metabolism. Cell Communication and Signaling: CCS , 11(1), 34. doi: 10.1186/1478-811X-11-34.
- Melrose, H. L., Dachsel, J. C., Behrouz, B., Lincoln, S. J., Yue, M., Hinkle, K. M., … Farrer, M. J. (2010). Impaired dopaminergic neurotransmission and microtubule-associated protein tau alterations in human LRRK2 transgenic mice. Neurobiology of Disease , 40(3), 503–517. doi: 10.1016/j.nbd.2010.07.010.
- Meredith, G. E., & Rademacher, D. J. (2011). MPTP mouse models of Parkinson's disease: An update. Journal of Parkinson's Disease , 1(1), 19–33. doi: 10.3233/JPD-2011-11023.
- Mishima, T., Fujioka, S., Fukae, J., Yuasa-Kawada, J., & Tsuboi, Y. (2018). Modeling Parkinson's disease and atypical parkinsonian syndromes using induced pluripotent stem cells. International Journal of Molecular Sciences , 19(12), E3870. doi: 10.3390/ijms19123870.
- Montoya, C. P., Campbell-Hope, L. J., Pemberton, K. D., & Dunnett, S. B. (1991). The “staircase test”: A measure of independent forelimb reaching and grasping abilities in rats. Journal of Neuroscience Methods , 36(2–3), 219–228. doi: 10.1016/0165-0270(91)90048-5.
- Moore, D. J., West, A. B., Dawson, V. L., & Dawson, T. M. (2005). Molecular pathophysiology of Parkinson's disease. Annual Review of Neuroscience , 28, 57–87. doi: 10.1146/annurev.neuro.28.061604.135718.
- Mosharov, E. V., Larsen, K. E., Kanter, E., Phillips, K. A., Wilson, K., Schmitz, Y., … Sulzer, D. (2009). Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron , 62(2), 218–229. doi: 10.1016/j.neuron.2009.01.033.
- Muma, N. A., Lee, J. M., Gorman, L., Heidenreich, B. A., Mitrovic, I., & Napier, T. C. (2001). 6-hydroxydopamine-induced lesions of dopaminergic neurons alter the function of postsynaptic cholinergic neurons without changing cytoskeletal proteins. Experimental Neurology , 168(1), 135–143. doi: 10.1006/exnr.2000.7582.
- Nalls, M. A., Pankratz, N., Lill, C. M., Do, C. B., Hernandez, D. G., Saad, M., … Singleton, A. B. (2014). Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson's disease. Nature Genetics , 46(9), 989–993. doi: 10.1038/ng.3043.
- Napolitano, A., Pezzella, A., & Prota, G. (1999). New reaction pathways of dopamine under oxidative stress conditions: Nonenzymatic iron-assisted conversion to norepinephrine and the neurotoxins 6-hydroxydopamine and 6, 7-dihydroxytetrahydroisoquinoline. Chemical Research in Toxicology , 12(11), 1090–1097. doi: 10.1021/tx990079p.
- Nedergaard, S., Flatman, J. A., & Engberg, I. (1993). Nifedipine- and omega-conotoxin-sensitive Ca2+ conductances in guinea-pig substantia nigra pars compacta neurones. Journal of Physiology , 466, 727–747.
- Nolbrant, S., Heuer, A., Parmar, M., & Kirkeby, A. (2017). Generation of high-purity human ventral midbrain dopaminergic progenitors for in vitro maturation and intracerebral transplantation. Nature Protocols , 12(9), 1962–1979. doi: 10.1038/nprot.2017.078.
- Nordstroma, U., Beauvais, G., Ghosh, A., Pulikkaparambil Sasidharan, B. C., Lundblad, M., Fuchs, J., … Brundin, P. (2015). Progressive nigrostriatal terminal dysfunction and degeneration in the engrailed1 heterozygous mouse model of Parkinson's disease. Neurobiology of Disease , 73, 70–82. doi: 10.1016/j.nbd.2014.09.012.
- Nouraei, N., Mason, D. M., Miner, K. M., Carcella, M. A., Bhatia, T. N., Dumm, B. K., … Leak, R. K. (2018). Critical appraisal of pathology transmission in the alpha-synuclein fibril model of Lewy body disorders. Experimental Neurology , 299(Pt A), 172–196. doi: 10.1016/j.expneurol.2017.10.017.
- Obeso, J. A., Rodriguez-Oroz, M. C., Goetz, C. G., Marin, C., Kordower, J. H., Rodriguez, M., … Halliday, G. (2010). Missing pieces in the Parkinson's disease puzzle. Nature Medicine , 16(6), 653–661. doi: 10.1038/nm.2165.
- Okuzumi, A., Kurosawa, M., Hatano, T., Takanashi, M., Nojiri, S., Fukuhara, T., … Nukina, N. (2018). Rapid dissemination of alpha-synuclein seeds through neural circuits in an in-vivo prion-like seeding experiment. Acta Neuropathologica Communications , 6(1), 96. doi: 10.1186/s40478-018-0587-0.
- Ormo, M., Cubitt, A. B., Kallio, K., Gross, L. A., Tsien, R. Y., & Remington, S. J. (1996). Crystal structure of the Aequorea victoria green fluorescent protein. Science , 273(5280), 1392–1395. doi: 10.1126/science.273.5280.1392.
- Oyama, G., Yoshimi, K., Natori, S., Chikaoka, Y., Ren, Y. R., Funayama, M., … Hattori, N. (2010). Impaired in vivo dopamine release in parkin knockout mice. Brain Research , 1352, 214–222. doi: 10.1016/j.brainres.2010.06.065.
- Pang, X., Hogan, E. M., Casserly, A., Gao, G., Gardner, P. D., & Tapper, A. R. (2014). Dicer expression is essential for adult midbrain dopaminergic neuron maintenance and survival. Molecular and Cellular Neuroscience , 58, 22–28. doi: 10.1016/j.mcn.2013.10.009.
- Papathanou, M., Dumas, S., Pettersson, H., Olson, L., & Wallen-Mackenzie, A. (2019). Off-target effects in transgenic mice: Characterization of dopamine transporter (DAT)-Cre transgenic mouse lines exposes multiple non-dopaminergic neuronal clusters available for selective targeting within limbic neurocircuitry. eNeuro , 6(5), ENEURO.0198–0119.2019. doi: 10.1523/ENEURO.0198-19.2019.
- Park, J. S., Davis, R. L., & Sue, C. M. (2018). Mitochondrial dysfunction in Parkinson's disease: New mechanistic insights and therapeutic perspectives. Current Neurology and Neuroscience Reports , 18(5), 21. doi: 10.1007/s11910-018-0829-3.
- Parkkinen, L., O'Sullivan, S. S., Collins, C., Petrie, A., Holton, J. L., Revesz, T., & Lees, A. J. (2011). Disentangling the relationship between lewy bodies and nigral neuronal loss in Parkinson's disease. Journal of Parkinson's Disease , 1(3), 277–286. doi: 10.3233/JPD-2011-11046.
- Peelaerts, W., Bousset, L., Van der Perren, A., Moskalyuk, A., Pulizzi, R., Giugliano, M., … Baekelandt, V. (2015). α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature , 522(7556), 340–344. doi: 10.1038/nature14547.
- Penttinen, A. M., Parkkinen, I., Blom, S., Kopra, J., Andressoo, J. O., Pitkanen, K., … Airavaara, M. (2018). Implementation of deep neural networks to count dopamine neurons in substantia nigra. European Journal of Neuroscience , 48(6), 2354–2361. doi: 10.1111/ejn.14129.
- Penttinen, A. M., Parkkinen, I., Voutilainen, M. H., Koskela, M., Back, S., Their, A., … Airavaara, M. (2018). Pre-alpha-pro-GDNF and pre-beta-pro-GDNF isoforms are neuroprotective in the 6-hydroxydopamine rat model of Parkinson's disease. Frontiers in Neurology , 9, 457. doi: 10.3389/fneur.2018.00457.
- Penttinen, A. M., Suleymanova, I., Albert, K., Anttila, J., Voutilainen, M. H., & Airavaara, M. (2016). Characterization of a new low-dose 6-hydroxydopamine model of Parkinson's disease in rat. Journal of Neuroscience Research , 94(4), 318–328. doi: 10.1002/jnr.23708.
- Perese, D. A., Ulman, J., Viola, J., Ewing, S. E., & Bankiewicz, K. S. (1989). A 6-hydroxydopamine-induced selective parkinsonian rat model. Brain Research , 494(2), 285–293. doi: 10.1016/0006-8993(89)90597-0.
- Perez, F. A., & Palmiter, R. D. (2005). Parkin-deficient mice are not a robust model of parkinsonism. Proceedings of the National Academy of Sciences of the United States of America , 102(6), 2174–2179. doi: 10.1073/pnas.0409598102.
- Petroske, E., Meredith, G. E., Callen, S., Totterdell, S., & Lau, Y. S. (2001). Mouse model of Parkinsonism: A comparison between subacute MPTP and chronic MPTP/probenecid treatment. Neuroscience , 106(3), 589–601. doi: 10.1016/s0306-4522(01)00295-0.
- Pickrell, A. M., Pinto, M., Hida, A., & Moraes, C. T. (2011). Striatal dysfunctions associated with mitochondrial DNA damage in dopaminergic neurons in a mouse model of Parkinson's disease. Journal of Neuroscience , 31(48), 17649–17658. doi: 10.1523/JNEUROSCI.4871-11.2011.
- Pinto, M., Nissanka, N., Peralta, S., Brambilla, R., Diaz, F., & Moraes, C. T. (2016). Pioglitazone ameliorates the phenotype of a novel Parkinson's disease mouse model by reducing neuroinflammation. Molecular Neurodegeneration , 11, 25. doi: 10.1186/s13024-016-0090-7.
- Planken, A., Porokuokka, L. L., Hanninen, A. L., Tuominen, R. K., & Andressoo, J. O. (2010). Medium-throughput computer aided micro-island method to assay embryonic dopaminergic neuron cultures in vitro. Journal of Neuroscience Methods , 194(1), 122–131. doi: 10.1016/j.jneumeth.2010.10.005.
- Polymeropoulos, M. H., Lavedan, C., Leroy, E., Ide, S. E., Dehejia, A., Dutra, A., … Nussbaum, R. L. (1997). Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science , 276(5321), 2045–2047. doi: 10.1126/science.276.5321.2045.
- Potashkin, J. A., Blume, S. R., & Runkle, N. K. (2010). Limitations of animal models of Parkinson's disease. Parkinson's Disease , 2011, 658083. doi: 10.4061/2011/658083.
- Przedborski, S., Levivier, M., Jiang, H., Ferreira, M., Jackson-Lewis, V., Donaldson, D., & Togasaki, D. M. (1995). Dose-dependent lesions of the dopaminergic nigrostriatal pathway induced by intrastriatal injection of 6-hydroxydopamine. Neuroscience , 67(3), 631–647. doi: 10.1016/0306-4522(95)00066-r.
- Ramonet, D., Daher, J. P., Lin, B. M., Stafa, K., Kim, J., Banerjee, R., … Moore, D. J. (2011). Dopaminergic neuronal loss, reduced neurite complexity and autophagic abnormalities in transgenic mice expressing G2019S mutant LRRK2. PLoS One , 6(4), e18568. doi: 10.1371/journal.pone.0018568.
- Ravenstijn, P. G., Merlini, M., Hameetman, M., Murray, T. K., Ward, M. A., Lewis, H., … de Lange, E. C. (2008). The exploration of rotenone as a toxin for inducing Parkinson's disease in rats, for application in BBB transport and PK-PD experiments. Journal of Pharmacological and Toxicological Methods , 57(2), 114–130. doi: 10.1016/j.vascn.2007.10.003.
- Rey, N. L., George, S., Steiner, J. A., Madaj, Z., Luk, K. C., Trojanowski, J. Q., … Brundin, P. (2018). Spread of aggregates after olfactory bulb injection of alpha-synuclein fibrils is associated with early neuronal loss and is reduced long term. Acta Neuropathologica , 135(1), 65–83. doi: 10.1007/s00401-017-1792-9.
- Rey, N. L., Steiner, J. A., Maroof, N., Luk, K. C., Madaj, Z., Trojanowski, J. Q., … Brundin, P. (2016). Widespread transneuronal propagation of alpha-synucleinopathy triggered in olfactory bulb mimics prodromal Parkinson's disease. Journal of Experimental Medicine , 213(9), 1759–1778. doi: 10.1084/jem.20160368.
- Rial, D., Castro, A. A., Machado, N., Garcao, P., Goncalves, F. Q., Silva, H. B., … Prediger, R. D. (2014). Behavioral phenotyping of Parkin-deficient mice: Looking for early preclinical features of Parkinson's disease. PLoS One , 9(12), e114216. doi: 10.1371/journal.pone.0114216.
- Richardson, J. R., Quan, Y., Sherer, T. B., Greenamyre, J. T., & Miller, G. W. (2005). Paraquat neurotoxicity is distinct from that of MPTP and rotenone. Toxicological Sciences , 88(1), 193–201. doi: 10.1093/toxsci/kfi304.
- Rieker, C., Engblom, D., Kreiner, G., Domanskyi, A., Schober, A., Stotz, S., … Parlato, R. (2011). Nucleolar disruption in dopaminergic neurons leads to oxidative damage and parkinsonism through repression of mammalian target of rapamycin signaling. Journal of Neuroscience , 31(2), 453–460. doi: 10.1523/JNEUROSCI.0590-10.2011.
- Rodriguez-Navarro, J. A., Casarejos, M. J., Menendez, J., Solano, R. M., Rodal, I., Gomez, A., … Mena, M. A. (2007). Mortality, oxidative stress and tau accumulation during ageing in parkin null mice. Journal of Neurochemistry , 103(1), 98–114. doi: 10.1111/j.1471-4159.2007.04762.x.
- Rousseaux, M. W., Marcogliese, P. C., Qu, D., Hewitt, S. J., Seang, S., Kim, R. H., … Park, D. S. (2012). Progressive dopaminergic cell loss with unilateral-to-bilateral progression in a genetic model of Parkinson disease. Proceedings of the National Academy of Sciences of the United States of America , 109(39), 15918–15923. doi: 10.1073/pnas.1205102109.
- Sakai, K., & Gash, D. M. (1994). Effect of bilateral 6-OHDA lesions of the substantia nigra on locomotor activity in the rat. Brain Research , 633(1–2), 144–150. doi: 10.1016/0006-8993(94)91533-4.
- Samaranch, L., Sebastian, W. S., Kells, A. P., Salegio, E. A., Heller, G., Bringas, J. R., … Bankiewicz, K. S. (2014). AAV9-mediated expression of a non-self protein in nonhuman primate central nervous system triggers widespread neuroinflammation driven by antigen-presenting cell transduction. Molecular Therapy , 22(2), 329–337. doi: 10.1038/mt.2013.266.
- Sanchez, G., Varaschin, R. K., Bueler, H., Marcogliese, P. C., Park, D. S., & Trudeau, L. E. (2014). Unaltered striatal dopamine release levels in young Parkin knockout, Pink1 knockout, DJ-1 knockout and LRRK2 R1441G transgenic mice. PLoS One , 9(4), e94826. doi: 10.1371/journal.pone.0094826.
- Sandlesh, P., Juang, T., Safina, A., Higgins, M. J., & Gurova, K. V. (2018). Uncovering the fine print of the CreERT2-LoxP system while generating a conditional knockout mouse model of Ssrp1 gene. PLoS One , 13(6), e0199785. doi: 10.1371/journal.pone.0199785.
- Sandor, C., Robertson, P., Lang, C., Heger, A., Booth, H., Vowles, J., … Webber, C. (2017). Transcriptomic profiling of purified patient-derived dopamine neurons identifies convergent perturbations and therapeutics for Parkinson's disease. Human Molecular Genetics , 26(3), 552–566. doi: 10.1093/hmg/ddw412.
- Saner, A., & Thoenen, H. (1971). Model experiments on the molecular mechanism of action of 6-hydroxydopamine. Molecular Pharmacology , 7(2), 147–154.
- Sauer, H., & Oertel, W. H. (1994). Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: A combined retrograde tracing and immunocytochemical study in the rat. Neuroscience , 59(2), 401–415. doi: 10.1016/0306-4522(94)90605-x.
- Savolainen, M. H., Albert, K., Airavaara, M., & Myohanen, T. T. (2017). Nigral injection of a proteasomal inhibitor, lactacystin, induces widespread glial cell activation and shows various phenotypes of Parkinson's disease in young and adult mouse. Experimental Brain Research Experimentelle Hirnforschung Experimentation Cerebrale , 235(7), 2189–2202. doi: 10.1007/s00221-017-4962-z.
- Schallert, T., Fleming, S. M., Leasure, J. L., Tillerson, J. L., & Bland, S. T. (2000). CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology , 39(5), 777–787. doi: 10.1016/s0028-3908(00)00005-8.
- Scherman, D., Desnos, C., Darchen, F., Pollak, P., Javoy-Agid, F., & Agid, Y. (1989). Striatal dopamine deficiency in Parkinson's disease: Role of aging. Annals of Neurology , 26(4), 551–557. doi: 10.1002/ana.410260409.
- Schmidt, S., Linnartz, B., Mendritzki, S., Sczepan, T., Lubbert, M., Stichel, C. C., & Lubbert, H. (2011). Genetic mouse models for Parkinson's disease display severe pathology in glial cell mitochondria. Human Molecular Genetics , 20(6), 1197–1211. doi: 10.1093/hmg/ddq564.
- Schober, A. (2004). Classic toxin-induced animal models of Parkinson's disease: 6-OHDA and MPTP. Cell and Tissue Research , 318(1), 215–224. doi: 10.1007/s00441-004-0938-y.
- Scholz, D., Poltl, D., Genewsky, A., Weng, M., Waldmann, T., Schildknecht, S., & Leist, M. (2011). Rapid, complete and large-scale generation of post-mitotic neurons from the human LUHMES cell line. Journal of Neurochemistry , 119(5), 957–971. doi: 10.1111/j.1471-4159.2011.07255.x.
- Senoh, S., & Witkop, B. (1959). Non-enzymatic conversions of dopamine to norepinephrine and trihydroxyphenethylamines. Journal of the American Chemical Society , 81(23), 6222–6231. doi: 10.1021/ja01532a028.
- Shahmoradian, S. H., Lewis, A. J., Genoud, C., Hench, J., Moors, T. E., Navarro, P. P., … Lauer, M. E. (2019). Lewy pathology in Parkinson's disease consists of crowded organelles and lipid membranes. Nature Neuroscience , 22(7), 1099–1109. doi: 10.1038/s41593-019-0423-2.
- Shan, L., Diaz, O., Zhang, Y., Ladenheim, B., Cadet, J. L., Chiang, Y. H., … Backman, C. M. (2015). L-Dopa induced dyskinesias in Parkinsonian mice: Disease severity or L-Dopa history. Brain Research , 1618, 261–269. doi: 10.1016/j.brainres.2015.06.005.
- Sherer, T. B., Betarbet, R., Testa, C. M., Seo, B. B., Richardson, J. R., Kim, J. H., … Greenamyre, J. T. (2003). Mechanism of toxicity in rotenone models of Parkinson's disease. Journal of Neuroscience , 23(34), 10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003.
- Shimizu, K., Ohtaki, K., Matsubara, K., Aoyama, K., Uezono, T., Saito, O., … Shiono, H. (2001). Carrier-mediated processes in blood–brain barrier penetration and neural uptake of paraquat. Brain Research , 906(1–2), 135–142. doi: 10.1016/s0006-8993(01)02577-x.
- Shimozawa, A., Ono, M., Takahara, D., Tarutani, A., Imura, S., Masuda-Suzukake, M., … Hasegawa, M. (2017). Propagation of pathological alpha-synuclein in marmoset brain. Acta Neuropathologica Communications , 5(1), 12. doi: 10.1186/s40478-017-0413-0.
- Smith, G. A., Heuer, A., Dunnett, S. B., & Lane, E. L. (2012). Unilateral nigrostriatal 6-hydroxydopamine lesions in mice II: Predicting l-DOPA-induced dyskinesia. Behavioural Brain Research , 226(1), 281–292. doi: 10.1016/j.bbr.2011.09.025.
- Smits, L. M., Reinhardt, L., Reinhardt, P., Glatza, M., Monzel, A. S., Stanslowsky, N., … Schwamborn, J. C. (2019). Modeling Parkinson's disease in midbrain-like organoids. NPJ Parkinson's Disease , 5, 5. doi: 10.1038/s41531-019-0078-4.
- Soto-Otero, R., Mendez-Alvarez, E., Hermida-Ameijeiras, A., Munoz-Patino, A. M., & Labandeira-Garcia, J. L. (2000). Autoxidation and neurotoxicity of 6-hydroxydopamine in the presence of some antioxidants: Potential implication in relation to the pathogenesis of Parkinson's disease. Journal of Neurochemistry , 74(4), 1605–1612. doi: 10.1046/j.1471-4159.2000.0741605.x.
- Specht, C. G., & Schoepfer, R. (2001). Deletion of the alpha-synuclein locus in a subpopulation of C57BL/6J inbred mice. BMC Neuroscience , 2, 11. doi: 10.1186/1471-2202-2-11.
- Spillantini, M. G., Crowther, R. A., Jakes, R., Hasegawa, M., & Goedert, M. (1998). Alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proceedings of the National Academy of Sciences of the United States of America , 95(11), 6469–6473. doi: 10.1073/pnas.95.11.6469.
- Stanic, D., Finkelstein, D. I., Bourke, D. W., Drago, J., & Horne, M. K. (2003). Timecourse of striatal re-innervation following lesions of dopaminergic SNpc neurons of the rat. European Journal of Neuroscience , 18(5), 1175–1188. doi: 10.1046/j.1460-9568.2003.02800.x.
- Stichel, C. C., Zhu, X. R., Bader, V., Linnartz, B., Schmidt, S., & Lubbert, H. (2007). Mono- and double-mutant mouse models of Parkinson's disease display severe mitochondrial damage. Human Molecular Genetics , 16(20), 2377–2393. doi: 10.1093/hmg/ddm083.
- Su, X., Fischer, D. L., Li, X., Bankiewicz, K., Sortwell, C. E., & Federoff, H. J. (2017). Alpha-synuclein mRNA is not increased in sporadic PD and alpha-synuclein accumulation does not block GDNF signaling in Parkinson's disease and disease models. Molecular Therapy , 25(10), 2231–2235. doi: 10.1016/j.ymthe.2017.04.018.
- Sulzer, D., Trudeau, L. E., & Rayport, S. (2008). Postnatally derived ventral midbrain dopamine neuron cultures as a model system for studying neurotoxicity and Parkinson's disease. In R. Nass & S. Przedborski (Eds.), Parkinson's disease: Molecular and therapeutic insights from model systems ( 1st ed., pp. 491–504). Amsterdam, The Netherlands: Academic Press. doi: 10.1016/B978-0-12-374028-1.X0001-2.
- Sundstrom, E., Stromberg, I., Tsutsumi, T., Olson, L., & Jonsson, G. (1987). Studies on the effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on central catecholamine neurons in C57BL/6 mice. Comparison with three other strains of mice. Brain Research , 405(1), 26–38. doi: 10.1016/0006-8993(87)90986-3.
- Surmeier, D. J., Obeso, J. A., & Halliday, G. M. (2017). Selective neuronal vulnerability in Parkinson disease. Nature Reviews Neuroscience , 18(2), 101–113. doi: 10.1038/nrn.2016.178.
- Tenenbaum, L., & Humbert-Claude, M. (2017). Glial cell line-derived neurotrophic factor gene delivery in Parkinson's disease: A delicate balance between neuroprotection, trophic effects, and unwanted compensatory mechanisms. Frontiers in Neuroanatomy , 11, 29. doi: 10.3389/fnana.2017.00029.
- Thakur, P., Breger, L. S., Lundblad, M., Wan, O. W., Mattsson, B., Luk, K. C., … Bjorklund, A. (2017). Modeling Parkinson's disease pathology by combination of fibril seeds and alpha-synuclein overexpression in the rat brain. Proceedings of the National Academy of Sciences of the United States of America , 114(39), E8284–E8293. doi: 10.1073/pnas.1710442114.
- Thiruchelvam, M., Brockel, B. J., Richfield, E. K., Baggs, R. B., & Cory-Slechta, D. A. (2000). Potentiated and preferential effects of combined paraquat and maneb on nigrostriatal dopamine systems: Environmental risk factors for Parkinson's disease? Brain Research , 873(2), 225–234. doi: 10.1016/s0006-8993(00)02496-3.
- Thomas, B., & Beal, M. F. (2007). Parkinson's disease. Human Molecular Genetics , 16(Spec No. 2), R183–R194. doi: 10.1093/hmg/ddm159.
- Tong, Y., Pisani, A., Martella, G., Karouani, M., Yamaguchi, H., Pothos, E. N., & Shen, J. (2009). R1441C mutation in LRRK2 impairs dopaminergic neurotransmission in mice. Proceedings of the National Academy of Sciences of the United States of America , 106(34), 14622–14627. doi: 10.1073/pnas.0906334106.
- Tsika, E., Kannan, M., Foo, C. S., Dikeman, D., Glauser, L., Gellhaar, S., … Moore, D. J. (2014). Conditional expression of Parkinson's disease-related R1441C LRRK2 in midbrain dopaminergic neurons of mice causes nuclear abnormalities without neurodegeneration. Neurobiology of Disease , 71, 345–358. doi: 10.1016/j.nbd.2014.08.027.
- Turiault, M., Parnaudeau, S., Milet, A., Parlato, R., Rouzeau, J. D., Lazar, M., & Tronche, F. (2007). Analysis of dopamine transporter gene expression pattern—generation of DAT-iCre transgenic mice. The FEBS Journal , 274(14), 3568–3577. doi: 10.1111/j.1742-4658.2007.05886.x.
- Uemura, N., Yagi, H., Uemura, M. T., Hatanaka, Y., Yamakado, H., & Takahashi, R. (2018). Inoculation of alpha-synuclein preformed fibrils into the mouse gastrointestinal tract induces Lewy body-like aggregates in the brainstem via the vagus nerve. Molecular Neurodegeneration , 13(1), 21. doi: 10.1186/s13024-018-0257-5.
- Ungerstedt, U. (1968). 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. European Journal of Pharmacology , 5(1), 107–110. doi: 10.1016/0014-2999(68)90164-7.
- Ungerstedt, U. (1971a). Adipsia and aphagia after 6-hydroxydopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiologica Scandinavica Supplementum , 367, 95–122. doi: 10.1111/j.1365-201X.1971.tb11001.x
- Ungerstedt, U. (1971b). Postsynaptic supersensitivity after 6-hydroxy-dopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiologica Scandinavica Supplementum , 367, 69–93. doi: 10.1111/j.1365-201X.1971.tb11000.x.
- Ungerstedt, U., & Arbuthnott, G. W. (1970). Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Research , 24(3), 485–493. doi: 10.1016/0006-8993(70)90187-3.
- Ungerstedt, U., Ljungberg, T., & Steg, G. (1974). Behavioral, physiological, and neurochemical changes after 6-hydroxydopamine-induced degeneration of the nigro-striatal dopamine neurons. Advances in Neurology , 5, 421–426.
- Valente, E. M., Arena, G., Torosantucci, L., & Gelmetti, V. (2012). Molecular pathways in sporadic PD. Parkinsonism & Related Disorders, 18(Suppl 1), S71–S73. doi: 10.1016/S1353-8020(11)70023-2.
- Venderova, K., & Park, D. S. (2012). Programmed cell death in Parkinson's disease. Cold Spring Harbor perspectives in medicine , 2(8), a009365. doi: 10.1101/cshperspect.a009365.
- Visanji, N. P., Brotchie, J. M., Kalia, L. V., Koprich, J. B., Tandon, A., Watts, J. C., & Lang, A. E. (2016). Alpha-synuclein-based animal models of Parkinson's disease: Challenges and opportunities in a new era. Trends in Neuroscience (Tins) , 39(11), 750–762. doi: 10.1016/j.tins.2016.09.003.
- Volpicelli-Daley, L. A., Luk, K. C., & Lee, V. M. (2014). Addition of exogenous alpha-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous alpha-synuclein to Lewy body and Lewy neurite-like aggregates. Nature Protocols , 9(9), 2135–2146. doi: 10.1038/nprot.2014.143.
- Volpicelli-Daley, L. A., Luk, K. C., Patel, T. P., Tanik, S. A., Riddle, D. M., Stieber, A., … Lee, V. M. (2011). Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron , 72(1), 57–71. doi: 10.1016/j.neuron.2011.08.033.
- Von Coelln, R., Thomas, B., Savitt, J. M., Lim, K. L., Sasaki, M., Hess, E. J., … Dawson, T. M. (2004). Loss of locus coeruleus neurons and reduced startle in parkin null mice. Proceedings of the National Academy of Sciences of the United States of America , 101(29), 10744–10749. doi: 10.1073/pnas.0401297101.
- Voutilainen, M. H., De Lorenzo, F., Stepanova, P., Back, S., Yu, L. Y., Lindholm, P., … Tuominen, R. K. (2017). Evidence for an additive neurorestorative effect of simultaneously administered CDNF and GDNF in hemiparkinsonian rats: Implications for different mechanism of action. eNeuro , 4(1), ENEURO.0117–0116.2017. doi: 10.1523/ENEURO.0117-16.2017.
- Wang, H. Q., Imai, Y., Inoue, H., Kataoka, A., Iita, S., Nukina, N., & Takahashi, R. (2008). Pael-R transgenic mice crossed with parkin deficient mice displayed progressive and selective catecholaminergic neuronal loss. Journal of Neurochemistry , 107(1), 171–185. doi: 10.1111/j.1471-4159.2008.05607.x.
- Wegrzynowicz, M., Bar-On, D., Calo, L., Anichtchik, O., Iovino, M., Xia, J., … Spillantini, M. G. (2019). Depopulation of dense alpha-synuclein aggregates is associated with rescue of dopamine neuron dysfunction and death in a new Parkinson's disease model. Acta Neuropathologica , 138(4), 575–595. doi: 10.1007/s00401-019-02023-x.
- Weintraub, D., & Burn, D. J. (2011). Parkinson's disease: The quintessential neuropsychiatric disorder. Movement Disorders , 26(6), 1022–1031. doi: 10.1002/mds.23664.
- Weng, Y. H., Chen, C. Y., Lin, K. J., Chen, Y. L., Yeh, T. H., Hsiao, I. T., … Wang, H. L. (2016). (R1441C) LRRK2 induces the degeneration of SN dopaminergic neurons and alters the expression of genes regulating neuronal survival in a transgenic mouse model. Experimental Neurology , 275(Pt 1), 104–115. doi: 10.1016/j.expneurol.2015.09.001.
- West, M. J., Slomianka, L., & Gundersen, H. J. (1991). Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anatomical Record , 231(4), 482–497. doi: 10.1002/ar.1092310411.
- Williams, L. R. (1996). Basal forebrain cholinergic lesions and complete transection of septal-hippocampal pathway. In J. R. Perez-Polo (Ed.), Paradigms of neural injury (pp. 106–114). San Diego, London: Academic Press.
- Windrem, M. S., Schanz, S. J., Morrow, C., Munir, J., Chandler-Militello, D., Wang, S., & Goldman, S. A. (2014). A competitive advantage by neonatally engrafted human glial progenitors yields mice whose brains are chimeric for human glia. Journal of Neuroscience , 34(48), 16153–16161. doi: 10.1523/JNEUROSCI.1510-14.2014.
- Xiong, H., Wang, D., Chen, L., Choo, Y. S., Ma, H., Tang, C., … Zhang, Z. (2009). Parkin, PINK1, and DJ-1 form a ubiquitin E3 ligase complex promoting unfolded protein degradation. Journal of Clinical Investigation , 119(3), 650–660. doi: 10.1172/JCI37617.
- Zeng, B. Y., Dass, B., Owen, A., Rose, S., Cannizzaro, C., Tel, B. C., & Jenner, P. (2002). 6-Hydroxydopamine lesioning differentially affects alpha-synuclein mRNA expression in the nucleus accumbens, striatum and substantia nigra of adult rats. Neuroscience Letters , 322(1), 33–36. doi: 10.1016/s0304-3940(02)00083-6.
- Zhang, L., Le, W., Xie, W., & Dani, J. A. (2012). Age-related changes in dopamine signaling in Nurr1 deficient mice as a model of Parkinson's disease. Neurobiology of Aging , 33(5), 1001.e7–16. doi: 10.1016/j.neurobiolaging.2011.03.022.
- Zhang, Y., Granholm, A. C., Huh, K., Shan, L., Diaz-Ruiz, O., Malik, N., … Backman, C. M. (2012). PTEN deletion enhances survival, neurite outgrowth and function of dopamine neuron grafts to MitoPark mice. Brain , 135(Pt 9), 2736–2749. doi: 10.1093/brain/aws196.
- Zhuang, X., Masson, J., Gingrich, J. A., Rayport, S., & Hen, R. (2005). Targeted gene expression in dopamine and serotonin neurons of the mouse brain. Journal of Neuroscience Methods , 143(1), 27–32. doi: 10.1016/j.jneumeth.2004.09.020.
Citing Literature
Number of times cited according to CrossRef: 40
- Laura Patricia Perez-Abshana, Miguel Mendivil-Perez, Marlene Jimenez-Del-Rio, Carlos Velez-Pardo, The GBA1 K198E Variant Is Associated with Suppression of Glucocerebrosidase Activity, Autophagy Impairment, Oxidative Stress, Mitochondrial Damage, and Apoptosis in Skin Fibroblasts, International Journal of Molecular Sciences, 10.3390/ijms25179220, 25 , 17, (9220), (2024).
- Zainah Al‐Qahtani, Hayder M. Al‐kuraishy, Ali I. Al‐Gareeb, Ali K. Albuhadily, Naif H. Ali, Athanasios Alexiou, Marios Papadakis, Hebatallah M. Saad, Gaber El‐Saber Batiha, The potential role of brain renin‐angiotensin system in the neuropathology of Parkinson disease: Friend, foe or turncoat?, Journal of Cellular and Molecular Medicine, 10.1111/jcmm.18495, 28 , 12, (2024).
- Naif H. Ali, Hayder M. Al‐Kuraishy, Ali I. Al‐Gareeb, Athanasios Alexiou, Marios Papadakis, Ali Abdullah AlAseeri, Mubarak Alruwaili, Hebatallah M. Saad, Gaber El‐Saber Batiha, BDNF/TrkB activators in Parkinson's disease: A new therapeutic strategy, Journal of Cellular and Molecular Medicine, 10.1111/jcmm.18368, 28 , 10, (2024).
- A. Amini, F. Esmaeili, M. Golpich, Possible role of lncRNAs in amelioration of Parkinson’s disease symptoms by transplantation of dopaminergic cells, npj Parkinson's Disease, 10.1038/s41531-024-00661-x, 10 , 1, (2024).
- Helike Lõhelaid, Mart Saarma, Mikko Airavaara, CDNF and ER stress: Pharmacology and therapeutic possibilities, Pharmacology & Therapeutics, 10.1016/j.pharmthera.2024.108594, 254 , (108594), (2024).
- Chinmay Pal, Targeting mitochondria with small molecules: A promising strategy for combating Parkinson’s disease, Mitochondrion, 10.1016/j.mito.2024.101971, 79 , (101971), (2024).
- Samuel Rantataro, Laura Ferrer Pascual, Tomi Laurila, Electrochemical detection of amine neurotransmitters is drastically different in buffer solutions, in vivo, and cell culture systems, Electrochemistry Communications, 10.1016/j.elecom.2024.107732, (107732), (2024).
- Sui He, Qin Ru, Lin Chen, Guodong Xu, Yuxiang Wu, Advances in animal models of Parkinson's disease, Brain Research Bulletin, 10.1016/j.brainresbull.2024.111024, 215 , (111024), (2024).
- Areej Turkistani, Hayder M. Al-kuraishy, Ali I. Al-Gareeb, Ali K. Albuhadily, Athanasios Alexiou, Marios Papadakis, Mohamed M. Elfiky, Hebatallah M. Saad, Gaber El-Saber Batiha, Therapeutic Potential Effect of Glycogen Synthase Kinase 3 Beta (GSK-3β) Inhibitors in Parkinson Disease: Exploring an Overlooked Avenue, Molecular Neurobiology, 10.1007/s12035-024-04003-z, 61 , 9, (7092-7108), (2024).
- Hayder M. Al-kuraishy, Esraa H. Fahad, Salah Al-Windy, Suzy A. El-Sherbeni, Walaa A. Negm, Gaber El-Saber Batiha, The effects of cholesterol and statins on Parkinson’s neuropathology: a narrative review, Inflammopharmacology, 10.1007/s10787-023-01400-z, 32 , 2, (917-925), (2024).
- Ganna Ameen, Basant Osama, Modeling Neural Circuits in Parkinson’s Disease, Handbook of Neurodegenerative Disorders, 10.1007/978-981-99-7557-0_46, (511-547), (2024).
- Yousef Rasmi, Ameneh Shokati, Shima Hatamkhani, Yeganeh Farnamian, Roya Naderi, Ladan Jalali, Assessment of the relationship between the dopaminergic pathway and severe acute respiratory syndrome coronavirus 2 infection, with related neuropathological features, and potential therapeutic approaches in COVID‐19 infection, Reviews in Medical Virology, 10.1002/rmv.2506, 34 , 1, (2024).
- Mei-Chou Lai, Wayne-Young Liu, Shorong-Shii Liou, I-Min Liu, Hispidin in the Medicinal Fungus Protects Dopaminergic Neurons from JNK Activation-Regulated Mitochondrial-Dependent Apoptosis in an MPP+-Induced In Vitro Model of Parkinson’s Disease, Nutrients, 10.3390/nu15030549, 15 , 3, (549), (2023).
- Engila Khan, Ikramul Hasan, M. Emdadul Haque, Parkinson’s Disease: Exploring Different Animal Model Systems, International Journal of Molecular Sciences, 10.3390/ijms24109088, 24 , 10, (9088), (2023).
- Władysław Lasoń, Danuta Jantas, Monika Leśkiewicz, Magdalena Regulska, Agnieszka Basta-Kaim, The Vitamin D Receptor as a Potential Target for the Treatment of Age-Related Neurodegenerative Diseases Such as Alzheimer’s and Parkinson’s Diseases: A Narrative Review, Cells, 10.3390/cells12040660, 12 , 4, (660), (2023).
- Rugmani Meenambal, Tomasz Kruk, Jacek Gurgul, Piotr Warszyński, Danuta Jantas, Neuroprotective effects of polyacrylic acid (PAA) conjugated cerium oxide against hydrogen peroxide- and 6-OHDA-induced SH-SY5Y cell damage, Scientific Reports, 10.1038/s41598-023-45318-6, 13 , 1, (2023).
- So Young Kim, Hyo Geun Choi, Yoo Hwan Kim, Mi Jung Kwon, Joo-Hee Kim, Heui Seung Lee, Ji Hee Kim, Longitudinal study of the inverse relationship between Parkinson’s disease and cancer in Korea, npj Parkinson's Disease, 10.1038/s41531-023-00562-5, 9 , 1, (2023).
- Abril Ramírez-Higuera, Carolina Peña-Montes, Alejandra Barroso-Hernández, Óscar López-Franco, Rosa María Oliart-Ros, Omega-3 polyunsaturated fatty acids and its use in Parkinson's disease, Treatments, Nutraceuticals, Supplements, and Herbal Medicine in Neurological Disorders, 10.1016/B978-0-323-90052-2.00016-0, (675-702), (2023).
- Naif H. Ali, Hayder M. Al-kuraishy, Ali I. Al-Gareeb, Saud A. Alnaaim, Hebatallah M. Saad, Gaber El-Saber Batiha, The Molecular Pathway of p75 Neurotrophin Receptor (p75NTR) in Parkinson’s Disease: The Way of New Inroads, Molecular Neurobiology, 10.1007/s12035-023-03727-8, 61 , 5, (2469-2480), (2023).
- Ganna Ameen, Basant Osama, Modeling Neural Circuits in Parkinson’s Disease, Handbook of Neurodegenerative Disorders, 10.1007/978-981-19-3949-5_46-1, (1-37), (2023).
- Anastasiia Kotliarova, Alexandra V. Podturkina, Alla V. Pavlova, Daria S. Gorina, Anastasiya V. Lastovka, Oleg V. Ardashov, Artem D. Rogachev, Arseniy E. Izyurov, Alla B. Arefieva, Alexander V. Kulikov, Tatyana G. Tolstikova, Konstantin P. Volcho, Nariman F. Salakhutdinov, Yulia Sidorova, A Newly Identified Monoterpenoid-Based Small Molecule Able to Support the Survival of Primary Cultured Dopamine Neurons and Alleviate MPTP-Induced Toxicity In Vivo, Molecules, 10.3390/molecules27238286, 27 , 23, (8286), (2022).
- Irena Hlushchuk, Justyna Barut, Mikko Airavaara, Kelvin Luk, Andrii Domanskyi, Piotr Chmielarz, Cell Culture Media, Unlike the Presence of Insulin, Affect α-Synuclein Aggregation in Dopaminergic Neurons, Biomolecules, 10.3390/biom12040563, 12 , 4, (563), (2022).
- Shivam Kumar Pandey, Rakesh Kumar Singh, Recent developments in nucleic acid-based therapies for Parkinson’s disease: Current status, clinical potential, and future strategies, Frontiers in Pharmacology, 10.3389/fphar.2022.986668, 13 , (2022).
- Jing Zhang, Bing Xue, Bin Jing, Huiling Tian, Naiwen Zhang, Mengyuan Li, Lihua Lu, Lin Chen, Huaqiong Diao, Yufei Chen, Min Wang, Xiaoli Li, LPS activates neuroinflammatory pathways to induce depression in Parkinson’s disease-like condition, Frontiers in Pharmacology, 10.3389/fphar.2022.961817, 13 , (2022).
- Ya-Kui Mou, Li-Na Guan, Xiao-Yan Yao, Jia-Hui Wang, Xiao-Yu Song, Yong-Qiang Ji, Chao Ren, Shi-Zhuang Wei, Application of Neurotoxin-Induced Animal Models in the Study of Parkinson’s Disease-Related Depression: Profile and Proposal, Frontiers in Aging Neuroscience, 10.3389/fnagi.2022.890512, 14 , (2022).
- Pik‐Shan Lo, Vladimir V. Rymar, Timothy E. Kennedy, Abbas F. Sadikot, The netrin‐1 receptor DCC promotes the survival of a subpopulation of midbrain dopaminergic neurons: Relevance for ageing and Parkinson’s disease, Journal of Neurochemistry, 10.1111/jnc.15579, 161 , 3, (254-265), (2022).
- Dale W. Prebble, Safak Er, Mingming Xu, Irena Hlushchuk, Andrii Domanskyi, Mikko Airavaara, Merrick G. Ekins, George D. Mellick, Anthony R. Carroll, α-synuclein aggregation inhibitory activity of the bromotyrosine derivatives aerothionin and aerophobin-2 from the subtropical marine sponge Aplysinella sp, Results in Chemistry, 10.1016/j.rechem.2022.100472, 4 , (100472), (2022).
- F. Gubinelli, G. Cazzolla, M. Negrini, I. Kulacz, A. Mehrdadian, G. Tomasello, C. Venuti, L. Sarauskyte, F. Jacobs, F.P. Manfredsson, M. Davidsson, A. Heuer, Lateralized deficits after unilateral AAV-vector based overexpression of alpha-synuclein in the midbrain of rats on drug-free behavioral tests, Behavioural Brain Research, 10.1016/j.bbr.2022.113887, 429 , (113887), (2022).
- Piotr Chmielarz, Andrii Domanskyi, Alpha-synuclein preformed fibrils: a tool to understand Parkinson’s disease and develop disease modifying therapy, Neural Regeneration Research, 10.4103/1673-5374.310686, 16 , 11, (2219), (2021).
- Sreesha Sree, Ilmari Parkkinen, Anna Their, Mikko Airavaara, Eija Jokitalo, Morphological Heterogeneity of the Endoplasmic Reticulum within Neurons and Its Implications in Neurodegeneration, Cells, 10.3390/cells10050970, 10 , 5, (970), (2021).
- Danuta Jantas, Władysław Lasoń, Preclinical Evidence for the Interplay between Oxidative Stress and RIP1-Dependent Cell Death in Neurodegeneration: State of the Art and Possible Therapeutic Implications, Antioxidants, 10.3390/antiox10101518, 10 , 10, (1518), (2021).
- Nicole K. Polinski, A Summary of Phenotypes Observed in the In Vivo Rodent Alpha-Synuclein Preformed Fibril Model, Journal of Parkinson's Disease, 10.3233/JPD-212847, 11 , 4, (1555-1567), (2021).
- Liu-Cheng Li, Jie Chen, Xiao-Bin Zhu, Meng Guo, Qin Chen, Hong-Mei Fang, Lian-Di Kan, Trends of complications in patients with Parkinson’s disease in seven major cities of China from 2016 to 2019, International Clinical Psychopharmacology, 10.1097/YIC.0000000000000370, 36 , 5, (274-278), (2021).
- Päivi Lindholm, Mart Saarma, Cerebral dopamine neurotrophic factor protects and repairs dopamine neurons by novel mechanism, Molecular Psychiatry, 10.1038/s41380-021-01394-6, 27 , 3, (1310-1321), (2021).
- Masato Ooka, Caitlin Lynch, Menghang Xia, Application of In Vitro Metabolism Activation in High-Throughput Screening, International Journal of Molecular Sciences, 10.3390/ijms21218182, 21 , 21, (8182), (2020).
- Maria Ejma, Natalia Madetko, Anna Brzecka, Konstanty Guranski, Piotr Alster, Marta Misiuk-Hojło, Siva G. Somasundaram, Cecil E. Kirkland, Gjumrakch Aliev, The Links between Parkinson’s Disease and Cancer, Biomedicines, 10.3390/biomedicines8100416, 8 , 10, (416), (2020).
- Danuta Jantas, Jakub Chwastek, Janusz Malarz, Anna Stojakowska, Władysław Lasoń, Neuroprotective Effects of Methyl Caffeate against Hydrogen Peroxide-Induced Cell Damage: Involvement of Caspase 3 and Cathepsin D Inhibition, Biomolecules, 10.3390/biom10111530, 10 , 11, (1530), (2020).
- Yulia A. Sidorova, Mart Saarma, Can Growth Factors Cure Parkinson’s Disease?, Trends in Pharmacological Sciences, 10.1016/j.tips.2020.09.010, 41 , 12, (909-922), (2020).
- Fatma Nihan Cankara, Caner Günaydın, Süleyman Sırrı Bilge, Özlem Özmen, Arjan Kortholt, The neuroprotective action of lenalidomide on rotenone model of Parkinson's Disease: Neurotrophic and supportive actions in the substantia nigra pars compacta, Neuroscience Letters, 10.1016/j.neulet.2020.135308, 738 , (135308), (2020).
- Weiwei Yang, Wenwen Hao, Zhuo Meng, Shiyan Ding, Xiaodi Li, Tao Zhang, Weixiao Huang, Lian Xu, Yu Zhang, Jian Yang, Xiaosong Gu, Molecular Regulatory Mechanism and Toxicology of Neurodegenerative Processes in MPTP/Probenecid-Induced Progressive Parkinson’s Disease Mice Model Revealed by Transcriptome, Molecular Neurobiology, 10.1007/s12035-020-02128-5, 58 , 2, (603-616), (2020).

