A Mouse Model of MC903-Induced Atopic Dermatitis
Md Jahangir Alam, Md Jahangir Alam, Liang Xie, Liang Xie, Yu-Anne Yap, Yu-Anne Yap, Remy Robert, Remy Robert
Abstract
Atopic dermatitis (AD) is a chronic, relapsing, and extremely pruritic inflammatory skin disease with a particular impact on children. AD pathogenesis is not yet fully understood, and there is no curative treatment for this disease. Therefore, several genetically or chemically-induced AD mouse models have been developed. These preclinical mouse models are an indispensable research tool for studying AD pathogenesis and evaluating the efficacy of new candidate AD therapeutics. A commonly used mouse model of AD has been developed using the topical application of a low-calcemic analog of vitamin D3, MC903, to induce AD-like inflammatory phenotypes that closely resemble human AD. Moreover, this model shows a minimal effect on systemic calcium metabolism that is observed in the vitamin D3-induced AD model. Thus, an expanding number of studies use the MC903-induced AD model to interrogate AD pathobiology in vivo and to test new candidate small molecule and monoclonal antibody therapies. This protocol describes in detail functional measurements including the measurement of skin thickness, which is a surrogate marker for ear skin inflammation, as well as itch assessment, histological evaluation to assess the structural changes associated with AD skin inflammation, and preparation of single-cell suspensions from ear skin and draining lymph nodes for the assessment of inflammatory leukocyte subset infiltration in these tissues using flow cytometry. © 2023 The Authors. Current Protocols published by Wiley Periodicals LLC.
Basic Protocol : Topical application of MC903 induces AD-like skin inflammation
Support Protocol 1 : Measurement of ear skin thickness
Support Protocol 2 : Itch assessment
Support Protocol 3 : Dissection of ear skin and ear draining lymph nodes
Support Protocol 4 : Histological evaluation and quantification
Support Protocol 5 : Preparation of single-cell suspension from ear skin and draining lymph nodes for the assessment of inflammatory immune cell infiltration using flow cytometry
INTRODUCTION
Atopic dermatitis (AD), also known as eczema, is a chronic relapsing inflammatory skin disease with a significant health burden worldwide, particularly in children (Alam, Xie, Yap, Marques, & Robert, 2022, Bieber, 2010, Eichenfield et al., 2014). The pathogenesis of AD has not yet been fully understood, and up to now, no curative therapy is available for this disease. Currently available treatments for AD are challenged by the limited armamentarium and high heterogeneity of the disease. Thus, mouse models are an indispensable preclinical tool to interrogate AD pathobiology in vivo and evaluate the efficacy of newly developed therapies for this disease. Consequently, several mouse models have been developed based on genetic modification and chemical treatment to induce human AD-like phenotypes.
A commonly used mouse model of AD has been developed by Li et al. in 2006 using a low-calcemic analog of vitamin D3, named MC903 (Li et al., 2006). This preclinical mouse model utilizes the topical application of MC903 on the skin, which induces skin and systemic AD-like inflammation. The phenotypes closely mimic most of the clinical, skin barrier-related, histological, and immunological characteristics observed in AD patients (Li et al., 2006). For instance, MC903 induces the activation and proliferation of epidermal keratinocytes and promotes the expression of thymic stromal lymphopoietin (TSLP) expression (Li et al., 2006), a master switch for Th2 allergic inflammation, including AD (Li et al., 2009, Yoo et al., 2005). Along with the induction of TSLP levels, other AD parameters are also observed upon MC903 treatment, such as epidermal hyperplasia and increased dermal infiltration of type 2 inflammatory immune cells, expression of Th2 cytokines, and elevated serum IgE (Alam et al., 2020, Li et al., 2006, Naidoo et al., 2018).
The MC903-induced AD model is straightforward to manage and does not involve any tricky experimental steps, hazardous agents like oxazolone or 2,4-dinitrofluorobenzene (DNFB), or complex genetic manipulations, which may take months-to-a-year for characterization and establishment. Another advantage of this model is that it does not affect systemic calcium metabolism in mice (Li et al., 2006). Therefore, it can be applied to the ear and dorsal skin at higher concentrations and for a longer time to induce AD inflammatory phenotypes (Li et al., 2006, Moosbrugger-Martinz, Schmuth, & Dubrac, 2017). Thus, this AD mouse model represents a highly convenient preclinical tool for exploring therapeutic avenues and the characterization of various aspects of AD pathogenesis from its onset to the fully established disease phenotype.
This article describes the MC903-induced AD mouse model using the topical application of MC903 (see Basic Protocol), skin inflammation assessment by measuring the thickening of ear skin using a dial thickness gauge (see Support Protocol 1) and assesses itching frequency which is a hallmark of AD (see Support Protocol 2). Support Protocol 3 outlines procedures for the dissection of ear skin and ear draining auricular lymph nodes. Support Protocol 4 describes histological staining of ear skin, histopathological evaluation and quantification of other AD phenotypes and structural changes associated with AD-skin inflammation, including epidermal hyperplasia (acanthosis) and thickening of the dermis, inflammatory cellular infiltrates in the dermis, parakeratosis (presence of nuclei in the corneocytes), and spongiosis (intercellular edema). Finally, Support Protocol 5 describes the preparation of a single-cell suspension from ear skin and draining lymph nodes to assess inflammatory immune cell infiltrations, which is a major index to measure the severity of skin inflammation using flow cytometry.
NOTE : All animal care and experimental protocols were approved by the Animal Ethics Committee of Monash University and conformed to national and institutional guidelines.
Basic Protocol: TOPICAL APPLICATION OF MC903 INDUCES AD-LIKE SKIN INFLAMMATION
Materials
-
Mice (C57BL/6J wild type, 6-10 weeks old)
-
Isoflurane (Baxter, cat. no. 100190360400)
-
MC903, calcipotriol, hydrate (Cayman Chemical, cat. no. 10009599)
-
Ethyl alcohol (EtOH, ethanol) , pure, 190 proof (Millipore Sigma, cat. no. EX0290)
-
37°C mouse heating pad
-
Precision-dial thickness gauge (Peacock, Ozaki Mfg. Co. Ltd, cat. no. GA-1)
-
High-precision laboratory scale
-
Additional reagents and equipment for Support Protocols 1–5.
Days 0-14: Induce dermatitis
1.Anesthetize mice using 4% isoflurane in 2 L/min of oxygen for induction and 2% isoflurane in 0.4 L/min of oxygen for maintenance (Fig. 1C and D).
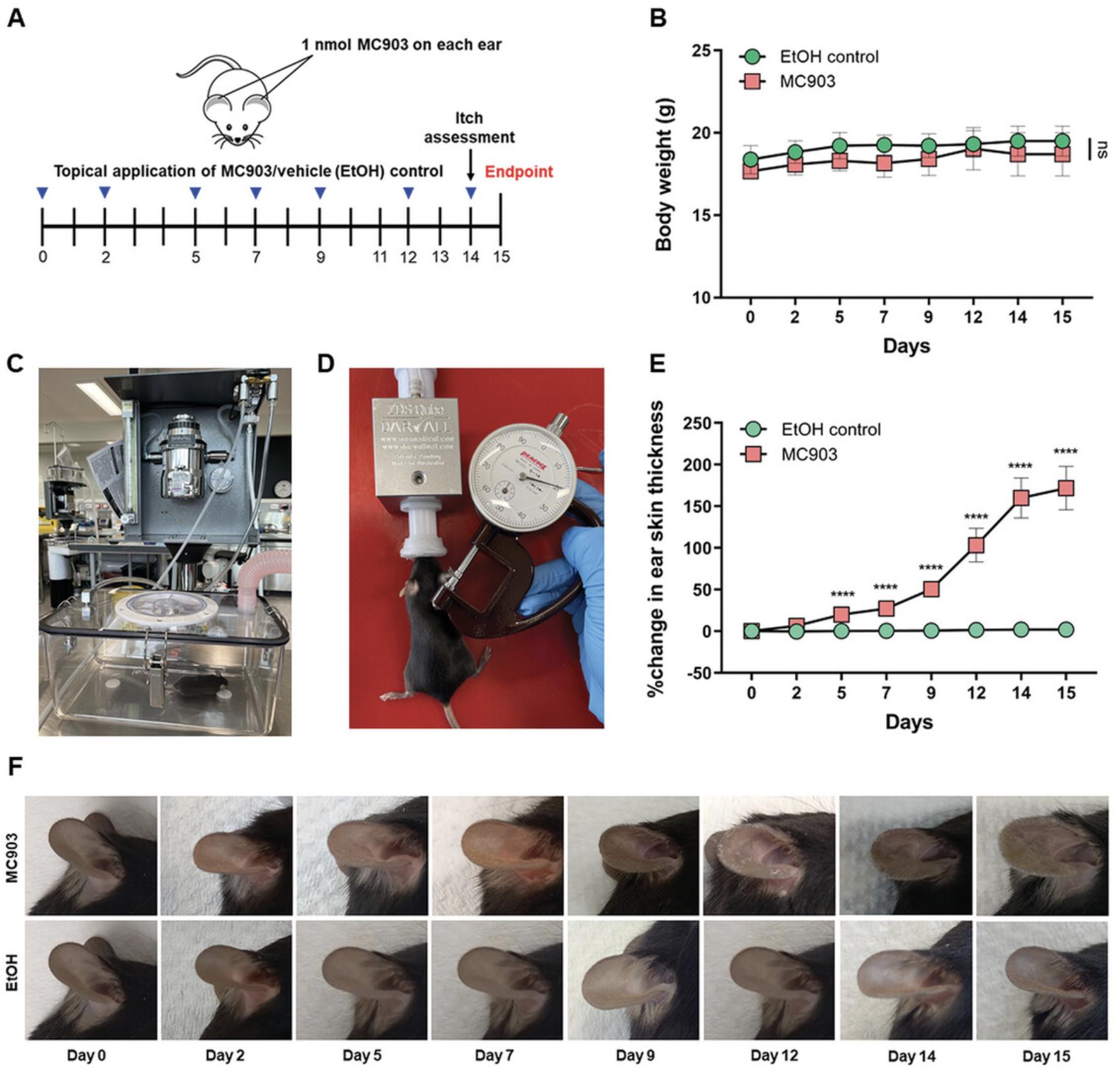
2.Weigh mice using a high-precision laboratory scale and place animals on a 37°C mouse heating pad to maintain body temperature. Record weights over the course of treatment (Fig. 1B).
3.Measure both left and right ear thickness using a precision-dial thickness gauge under light anesthesia (Fig. 1D, see Support Protocol 1).
4.Topically, apply 1 nM MC903 in 100% absolute ethyl alcohol (EtOH) using a 2-20 µl pipette to both sides of each ear (10 μl on dorsal and ventral ear sides for a total volume of 20 μl per ear) on every treatment day according to the treatment regimen (Fig. 1A). Control mice are treated in parallel with the same volume of EtOH (as a vehicle control) only.
5.After the treatment, keep animals on a 37°C mouse heating pad to maintain constant body temperature during recovery, then return to cages.
6.Over the next 2 weeks (see treatment regimen, Fig. 1A), repeat measurements before topical application of MC903 or EtOH (steps 2-5). A total of 7 applications of MC903 or EtOH will be applied, with the final topical application on day 14.
Day 14: Itch assessment
7.On day 14, 24 hr before the experimental endpoint, record a video of the mice and quantify itch events via time-lapse videography. Determine and quantify itch events for 30 min (see Support Protocol 2).
Day 15: Experimental endpoint
8.At the experimental endpoint (day 15), euthanize mice by CO2 asphyxiation, repeat measurements (step 3), and harvest ear skin tissues using sterile sharp scissors and forceps (Alam et al., 2020, Mac-Daniel, Buckwalter, Gueirard, & Ménard, 2016). For ear draining lymph nodes, carefully cut the skin at the corresponding neck region and dissect the auricular lymph nodes (Alam et al., 2020, Mac-Daniel et al., 2016) (see Support Protocols 3–5).
Support Protocol 1: MEASUREMENT OF EAR SKIN THICKNESS
The topical application of MC903 can induce AD-like skin inflammation, and an increase in the thickening of ear skin is considered a surrogate marker for skin inflammation (Alam et al., 2020, Naidoo et al., 2018, Moosbrugger-Martinz et al., 2017). The time course of ear skin inflammation can be monitored by measuring the ear skin thickness with a dial thickness gauge (Alam et al., 2020, Naidoo et al., 2018, Xie et al., 2021).
Materials
-
70% (v/v) ethanol
-
Precision-dial thickness gauge (Peacock, Ozaki Mfg. Co. Ltd, cat. no. GA-1)
1.Thoroughly wipe the dial thickness gauge with 70% ethanol. Place anesthetized animals on a 37°C mouse heating pad to maintain constant body temperature (see Basic Protocol). Measure and record both the right and left ear skin thickness with a dial thickness gauge (Fig. 1D).
2.On each treatment day, repeat measurements before the MC903 or EtOH treatment.
3.Calculate and plot the percent increase in skin thickness compared with day 0 baseline values (Fig. 1E).
Support Protocol 2: ITCH ASSESSMENT
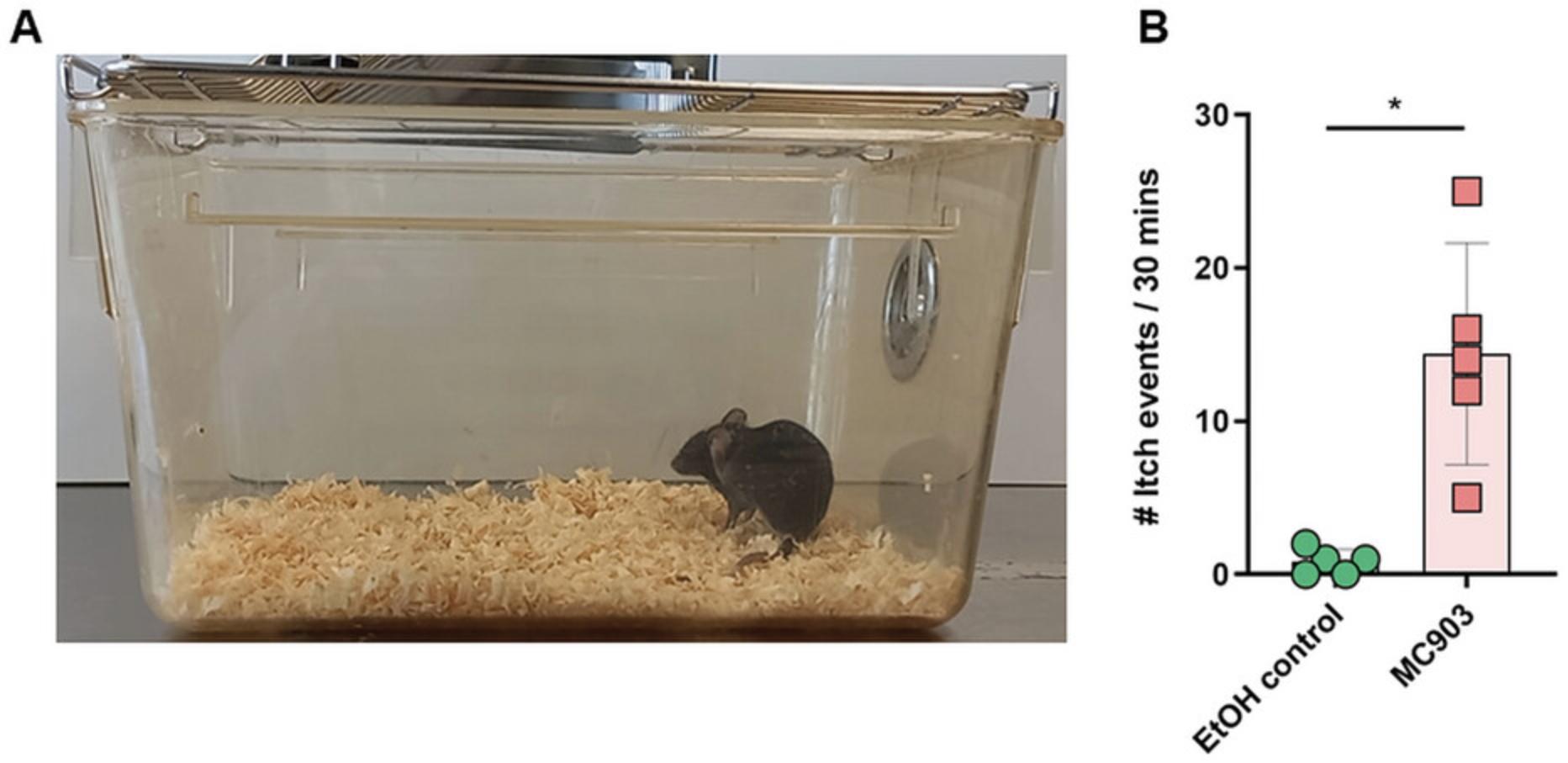
Itching is one of the hallmarks of AD, and itch-evoked scratching increases because of the MC903 treatment (Alam et al., 2020, Naidoo et al., 2018). Itch frequency is determined and quantified on a recorded video captured at 24 hr before the experimental endpoint, for a period of 30 min.
Materials
- Microisolator cage type II
- Video recorder
1.On day 14, place mice in a clear Microisolator type II cage (Fig. 2A). Record a video via time-lapse videography and quantify itch events for 30 min (Fig. 2B).
Support Protocol 3: DISSECTION OF EAR SKIN AND EAR DRAINING LYMPH NODES
Materials
-
RPMI 1640 medium, no glutamine (Gibco, cat. no. 21870076)
-
FBS (Sigma-Aldrich, cat. no. C8056)
-
R-10 medium (see recipe)
-
Phosphate buffered saline (PBS), sterile (Sigma-Aldrich, cat. no. D8537)
-
70% (v/v) ethanol
-
5-ml flat-bottom tubes (Techno Plas, cat. no. P5016SU)
-
1.5-ml Eppendorf tubes
-
Needles, 0.5 × 16 mm
-
Forceps, sterile, surgical
-
Scissors, sterile, surgical
-
Ice bucket
1.Prepare 5-ml flat-bottom tubes; add 2 ml of R-10 medium into each tube for skin samples. Prepare 1.5-ml Eppendorf tubes; add 1 ml of PBS per tube for ear draining lymph nodes.
2.At the experimental endpoint, euthanize mice by CO2 asphyxiation and place them on a dissecting board. Extend the mouse on its back with the limbs spread and fixed with needles in the four paws.
3.Wet fur and the corresponding neck region with 70% ethanol to avoid possible contamination.
4.Harvest one ear using sterile sharp scissors and forceps and separate the dorsal and ventral layers using two forceps, starting at the cut edge (Fig. 3C). Place them into a 5-ml flat-bottom tube containing 2 ml of R-10 medium; make sure both tissues are completely immersed in the medium. Place them on ice until further processing.
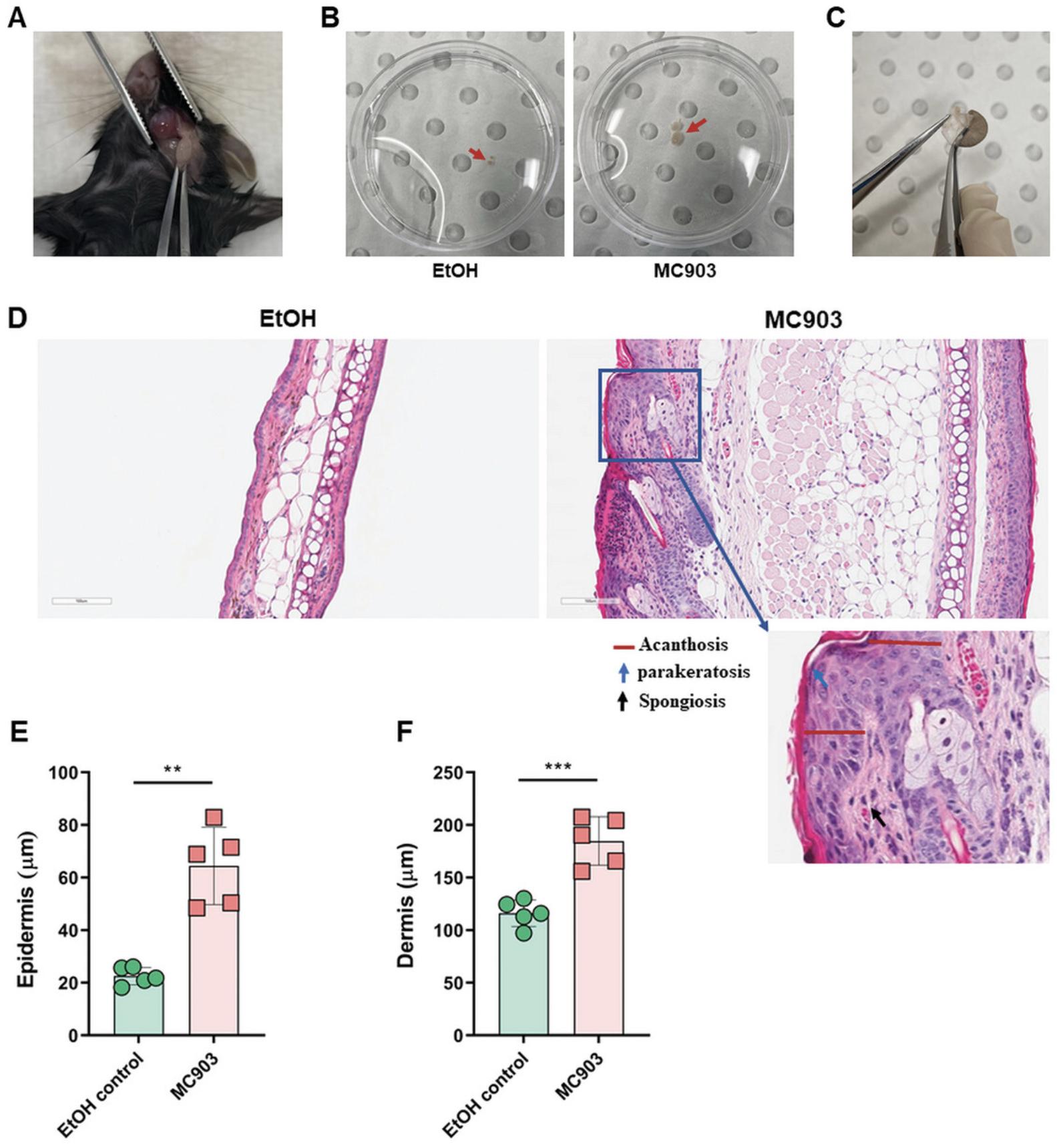
5.Using forceps and scissors, carefully cut the skin at the corresponding neck region and dissect the ear draining auricular lymph nodes (Fig. 3A). Place the lymph nodes into a 1.5-ml Eppendorf tube containing 1 ml of PBS, store on ice until further processing.
Support Protocol 4: HISTOLOGICAL EVALUATION AND QUANTIFICATION
An important characteristic of the MC903-induced atopic dermatitis mouse model is the ability to recapitulate the structural changes associated with AD skin inflammation, including acanthosis, thickening of the dermis, parakeratosis, presence of spongiosis, and inflammatory lymphocyte infiltrates in the dermis (Alam et al., 2020, Li et al., 2006, Moosbrugger-Martinz et al., 2017) (Fig. 3D, E, and F).
Materials
-
10% neutral buffered formalin
-
Hematoxylin-eosin (H&E) solution
-
Forceps, sterile, surgical
-
Scissors, sterile, surgical
-
Microscope slides
-
Aperio ImageScope software
-
ImageJ software (National Institutes of Health)
-
Additional reagents and equipment for standard methods of sectioning fixed tissue and staining with H&E (Moosbrugger-Martinz et al., 2017).
1.After sacrificing animals by CO2 asphyxiation, excise the tissue of one ear using sterile forceps and scissors. Fix it in 10% neutral buffered formalin for 24 hr.
2.Embed one ear in paraffin, cut three 4-μm sections with a microtome, place sections on microscope slides and stain with H&E according to standard procedures (Moosbrugger-Martinz et al., 2017).
3.Scan and capture images of H&E stained tissue slides at 20× magnification using the Aperio ImageScope software.
4.Measure epidermal and dermal thickness at three separate sites in the field of view on each tissue section using ImageJ software.
5.Calculate and plot the average of the collected data (Fig. 3E and F).
Support Protocol 5: PREPARATION OF SINGLE-CELL SUSPENSION FROM EAR SKIN AND DRAINING LYMPH NODES FOR THE ASSESSMENT OF INFLAMMATORY IMMUNE CELL INFILTRATION USING FLOW CYTOMETRY
Materials
-
RPMI 1640 medium, no glutamine (Gibco, cat. no. 21870076)
-
FBS (Sigma-Aldrich, cat. no. C8056)
-
R-10 medium (see recipe)
-
Collagenase, type IV (Gibco, cat. no. 17104-019)
-
DNase I, grade II, from bovine pancreas (Roche, cat. no. 10104159001)
-
2× digestion medium (see recipe)
-
0.5 M EDTA (Invitrogen, cat. no. 15575020)
-
Buffer 1 (see recipe)
-
Phosphate buffered saline (PBS), sterile (Sigma-Aldrich, cat. no. D8537)
-
FACS buffer (see recipe)
-
Scissors, sterile, surgical
-
5-ml flat-bottom tubes (Techno Plas, cat. no. P5016SU)
-
Ice bucket
-
50-ml conical tubes
-
Disposable nylon cell strainers (mesh, size 70 μm)
-
1-ml syringe
-
1-ml pipette and tips
-
Shaker-incubator
Single-cell suspension from ear skin
1.Chop ear leaflets into fine pieces with sharp scissors to facilitate tissue digestion inside the 5-ml flat-bottom tube containing 2 ml of R-10 medium.
2.After mechanical disruption of ear leaflets into fine pieces, add 2 ml of 2× digestion medium into each tube and incubate in a thermo-shaker incubator at 37°C for 20 min under 100 rpm agitation.
3.Add 1 ml of Buffer 1 into each tube to neutralize enzyme (collagenase IV and DNase I) activity. Perform the following steps on ice.
4.Prepare a single-cell suspension by filtering all ear tissue fragments and solution into a 50-ml falcon tube containing 5 ml of Buffer 1 through a 70-μm cell strainer and dissociating the remaining tissue fragments using a syringe plunger. Press the syringe plunger in circular motions against the strainer until all that remains is white connective tissue.
5.Rinse the strainers with 5 ml of R-10 medium to ensure all cells are collected. Centrifuge the tube for 5 min at 400 × g at 4°C and then discard the supernatant.
6.Wash the cell pellet with 10 ml of FACS buffer, centrifuge for 5 min at 400 × g at 4°C and then discard the supernatant.
7.Resuspend the cell pellet in FACS buffer, keep the tube on ice for antibody staining and flow cytometry analysis.
Single-cell suspension from lymph nodes
8.Mechanically disrupt and dissociate lymph nodes using the flat end of a 1 ml syringe plunger. Press the syringe plunger in circular motions against the 70 μm cell strainer until all that remains is white connective tissues.
9.Rinse the cell strainer with 5 ml of FACS buffer to ensure all cells are collected and centrifuge for 5 min at 400 × g at 4°C.
10.Discard the supernatant and resuspend the cell pellet in FACS buffer, keep the tube on ice for antibody staining and flow cytometry analysis.
REAGENTS AND SOLUTIONS
All media and buffer solutions should be sterile filtered using a 0.2 µm membrane filter prior to use.
Buffer 1
- R-10 medium (see recipe) containing 5 mM EDTA
Digestion medium (2×)
- R-10 medium (see recipe) containing 240 µg/ml of collagenase type IV and 4 mg/ml of DNase I.
(Final concentration of collagenase, type IV is 2 mg/ml, and DNase I is 120 µg/ml)
FACS buffer composition
- PBS containing 2% FBS and 4 mM EDTA
R-10 medium
- RPMI-1640 medium supplemented with 10% FBS
COMMENTARY
Background Information
The topical application of MC903, a low-calcemic analog of vitamin D3, induces skin inflammation and changes in skin morphology which closely resemble inflammation associated with human AD (Alam et al., 2020, Li et al., 2006, Naidoo et al., 2018). Other AD characteristics, such as skin eczematous-like lesions with xerosis and pruritus, increased serum IgE levels, inflammatory infiltrates in the skin and draining lymph nodes, were also observed in mice treated with this compound (Li et al., 2006, Naidoo et al., 2018). Interestingly, these AD symptoms are independent of mouse gender or genetic background (Moosbrugger-Martinz et al., 2017). Moreover, this mouse model is straightforward to manage and does not involve any tricky experimental steps such as tape stripping and occlusive patches, hazardous agents like DNFB, or complex genetic manipulations that may take months to a year for characterization and establishment. Additionally, MC903 has minimal effects on systemic calcium metabolism (Kragballe, 1992) and can be applied to mouse ears as well as in the dorsal skin after hair removal at higher concentrations and for a longer time (Li et al., 2006, Moosbrugger-Martinz et al., 2017). No other health impairments and weight loss were observed because of MC903 treatment. Thus, the MC903-induced AD mouse model is a promising preclinical research tool for studying immunologic abnormalities involved in AD development or maintenance or preclinical studies for unravelling new therapeutic strategies for this disease.
Critical Parameters and Troubleshooting
The storage condition for MC903 is -20°C, with protection from light. After reconstitution in absolute EtOH, aliquot and store them in safe lock tubes sealed with parafilm to avoid ethanol evaporation. The working solutions of MC903 should be prepared to be used only once to avoid air oxidation and evaporation of the ethanol.
During the MC903 treatment, place anesthetized animals on a 37°C mouse heating pad to maintain body temperature. To help mice recover from anesthesia, keep animals on a 37°C mouse heating pad to maintain constant body temperature during recovery, then return to their home cages. This recovery procedure should not exceed 5 to 10 min.
To evaluate skin inflammation, measuring skin thickness in the same skin area on each treatment day is critical. Importantly, gently measure the ear skin's thickness with little-to-no compression.
Understanding Results
The application of MC903 topically on the ear induces skin inflammation and other AD-like phenotypes in C57BL/6J wild type (WT) mice. This treatment increases the thickening of ear skin, which is a surrogate marker of ear skin inflammation. During the first week of the MC903 treatment, only minor changes in ear appearance are visible such as redness, dryness, swelling, and hyper-vascularization. These changes are clearly observed from day 5 of the MC903 treatment and worsen over time, particularly during the last days of the experiment. Ear draining lymph nodes also enlarge after MC903 treatment (Fig. 3B). However, EtOH (as vehicle control) treated ears did not show any changes. No other health impairments or weight loss were observed in this model (Fig. 1B). This experiment should be repeated at least three times, with at least five mice per group, to obtain the most robust and reliable results.
Time Considerations
In this model, a single experiment requires fifteen days to complete. These include seven days (day 0, 2, 5, 7, 9, 12, and 14) of the MC903 or EtOH treatments, monitoring and evaluating skin inflammation, and measuring body weight. One day (day 14) for video recording (30 min) and itch assessment. The experimental endpoint (day 15) is the final day to complete measurements and obtain tissue samples for subsequent analysis.
The measurement of body weight, ear skin thickness, and MC903 or EtOH treatments on the ear skin requires 30 to 45 min per six mice. For itch assessment, record a video for 30 min. Dissection of ear skin and draining lymph nodes requires 60 to 70 min per six mice. Single-cell preparation from ear tissues and draining lymph nodes requires 90 min. Processing excised ear skin samples for histological staining takes four days, and histological analysis takes 10 to 15 min per slide.
Acknowledgments
M.J.A. and L.X. are supported by the Monash Graduate Scholarships. The authors acknowledge the Monash Histology Platform for assistance with histology analysis.
Open access publishing facilitated by Monash University, as part of the Wiley - Monash University agreement via the Council of Australian University Librarians.
Author Contributions
Md Jahangir Alam : Methodology, validation, writing - original draft, writing – review, and editing. Liang Xie : Methodology, writing – review, and editing. Yu-Anne Yap : Data curation, investigation, methodology, writing – review, and editing. Remy Robert : Funding acquisition, project administration, resources, supervision, writing – review, and editing.
Conflict of Interest
The authors declare that they do not have any conflicts of interests.
Open Research
Data Availability Statement
The author has provided the required Data Availability Statement, and if applicable, included functional and accurate links to said data therein.
Literature Cited
- Alam, M. J., Xie, L., Ang, C., Fahimi, F., Willingham, S. B., Kueh, A. J., … Robert, R. (2020). Therapeutic blockade of CXCR2 rapidly clears inflammation in arthritis and atopic dermatitis models: Demonstration with surrogate and humanized antibodies. MAbs , 12(1), 1856460. doi: 10.1080/19420862.2020.1856460
- Alam, M. J., Xie, L., Yap, Y.-A., Marques, F. Z., & Robert, R. (2022). Manipulating microbiota to treat atopic dermatitis: Functions and therapies. Pathogens , 11(6), 642. doi: 10.3390/pathogens11060642
- Bieber, T. (2010). Atopic dermatitis. Annals of Dermatology , 22(2), 125–137. doi: 10.5021/ad.2010.22.2.125
- Eichenfield, L. F., Tom, W. L., Chamlin, S. L., Feldman, S. R., Hanifin, J. M., Simpson, E., … Sidbury, R. (2014). Guidelines of care for the management of atopic dermatitis: Section 1. Diagnosis and assessment of atopic dermatitis. Journal of the American Academy of Dermatology , 70(2), 338–351. doi: 10.1016/j.jaad.2013.10.010
- Kragballe, K. (1992). Vitamin D analogues in the treatment of psoriasis. Journal of Cellular Biochemistry , 49(1), 46–52. doi: 10.1002/jcb.240490109
- Lee, J. J., Protheroe, C. A., Luo, H., Ochkur, S. I., Scott, G. D., Zellner, K. R., … Lee, N. A. (2015). Eosinophil-dependent skin innervation and itching following contact toxicant exposure in mice. Journal of Allergy and Clinical Immunology , 135(2), 477–487. doi: 10.1016/j.jaci.2014.07.003
- Li, M., Hener, P., Zhang, Z., Ganti, K. P., & Metzger, D. (2009). Induction of thymic stromal lymphopoietin expression in keratinocytes is necessary for generating an atopic dermatitis upon application of the active vitamin D3 analogue MC903 on mouse skin. Journal of Investigative Dermatology , 129(2), 498–502. doi: 10.1038/jid.2008.232
- Li, M., Hener, P., Zhang, Z., Kato, S., Metzger, D., & Chambon, P. (2006). Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proceedings of the National Academy of Science USA , 103(31), 11736–11741. doi: 10.1073/pnas.0604575103
- Mac-Daniel, L., Buckwalter, M. R., Gueirard, P., & Ménard, R. (2016). Myeloid cell isolation from mouse skin and draining lymph node following intradermal immunization with live attenuated plasmodium sporozoites. Journal of Visualized Experiments: JoVE , (111), 53796. doi: 10.3791/53796
- Moosbrugger-Martinz, V., Schmuth, M., & Dubrac, S. (2017). A mouse model for atopic dermatitis using topical application of vitamin D3 or of its analog MC903. Methods in Molecular Biology , 1559, 91–106. doi: 10.1007/978-1-4939-6786-5_8
- Naidoo, K., Jagot, F., van den Elsen, L., Pellefigues, C., Jones, A., Luo, H., … Forbes-Blom, E. (2018). Eosinophils determine dermal thickening and water loss in an MC903 model of atopic dermatitis. Journal of Investigative Dermatology , 138(12), 2606–2616. doi: 10.1016/j.jid.2018.06.168
- Xie, L., McKenzie, C. I., Qu, X., Mu, Y., Wang, Q., & Bing, N. (2021). pH and proton sensor GPR65 determine susceptibility to atopic dermatitis. Journal of Immunology , 207(1), 101–109. doi: 10.4049/jimmunol.2001363
- Yoo, J., Omori, M., Gyarmati, D., Zhou, B., Aye, T., Brewer, A., … Ziegler, S. F. (2005). Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. Journal of Experimental Medicine , 202(4), 541–549. doi: 10.1084/jem.20041503
Citing Literature
Number of times cited according to CrossRef: 3
- Xin Ma, Guoshu Deng, Na Tian, Hao Wang, Hang Zhao, Le Kuai, Ying Luo, Chunjie Gao, Xiaojie Ding, Bin Li, Bin Li, Calycosin enhances Treg differentiation for alleviating skin inflammation in atopic dermatitis, Journal of Ethnopharmacology, 10.1016/j.jep.2024.117883, 326 , (117883), (2024).
- Cheng Lu, ShiJun Deng, YanJiao Liu, ShengJin Yang, DingMei Qin, LiJuan Zhang, Rui-rui Wang, Yi Zhang, Inhibition of macrophage MAPK/NF-κB pathway and Th2 axis by mangiferin ameliorates MC903-induced atopic dermatitis, International Immunopharmacology, 10.1016/j.intimp.2024.112038, 133 , (112038), (2024).
- Rou Zheng, Yan Ren, Xinyue Liu, Canxia He, Hua Liu, Yixuan Wang, Jianing Li, Shuya Xia, Zhifang Liu, Yizhao Ma, Dianchen Wang, Suling Xu, Geng Wang, Na Li, Exogenous drug-induced mouse models of atopic dermatitis, Cytokine & Growth Factor Reviews, 10.1016/j.cytogfr.2024.01.003, 77 , (104-116), (2024).

