Synthetic Procedure of 2-(1-(4-hydroxy-3-methoxyphenyl)propan-2-yl)-6-methoxy-4-propylphenol
Lisa.Stanley, Rui Katahira, Gregg T. Beckham
Disclaimer
This work was authored by the National Renewable Energy Laboratory, operated by Alliance for Sustainable Energy, LLC, for the U.S. Department of Energy (DOE) under Contract No. DE-AC36-08GO28308. Funding provided by U.S. Department of Energy Office of Energy Efficiency and Renewable Energy Bioenergy Technologies Office. The views expressed herein do not necessarily represent the views of the DOE or the U.S. Government.
Abstract
A direct understanding of the degradation reaction pathways of lignin polymers in biomass is difficult due to the complexity of lignin’s structure. To overcome the difficulty, simple lignin dimeric and trimeric model compounds which include typical lignin interunit linkages are useful to clarify reaction mechanisms. The following procedure describes the synthetic procedure of a β-5 dimeric lignin model compound: 2-(1-(4-hydroxy-3-methoxyphenyl)propan-2-yl)-6-methoxy-4-propylphenol. Lignin model compounds are useful for screening the effectiveness of catalysts and microoganisms. As well as determining the effect of a treatment on the lignin fraction, in particular the effect on the degree of depolymerization in the lignin polymer.
Before start
All glassware is dried in an oven set to 105ºC then cooled in a desiccator prior to use.
Steps
Synthetic Procedure
A solution of 1480mL citrate-phosphate buffer (20 mM, pH 3.5) [see Note 1] was heated to 38°C in a silicone oil bath. A solution of isoeugenol (5.00mL , 0.0328 mol) in methanol (164mL ) was added in portions with vigorous stirring to the buffer solution. 20mg horseradish peroxidase (HRP, 2500 U, Type II) was then added to the solution. The mixture was stirred while hydrogen peroxide, H2O2, (1.875mL , 0.0612 mol) was added dropwise over 10 min. The reaction mixture was stirred for an additional 1 h and then filtered using a Büchner funnel. The resulting residue was separated and the funnel was washed with ethyl acetate (EtOAc). The organic solubles and the residue were combined and washed with a saturated solution of brine [see Note 2], and then dried using sodium sulfate (Na2SO4).[1,2] The crude product obtained after evaporation of the solvent in vacuo was crystallized from methanol to afford (±)-licarin A (1.3176 g, 24.6%).
[1]
Licarin a (1.21g , 3.71 mmol) was charged into a round-bottom flask and dissolved in methanol (45mL) . 0.45g 5 wt% Palladium on carbon (Pd-C) was added gently to the reaction mixture followed by 4.66mL hydrochloric acid.
The reaction was then stirred at room temperature under a hydrogen filled balloon for 44 hours. After which is was filtered and concentrated in vacuo .[3] Crude mixture was purified via flash chromatography to yield the final product as a light yellow oil (0.6785 g, 55.4%).
[3]
Purification
Flash chromatography was performed using a Teledyne Isco Combiflash® NextGen 300+. Collected fractions were determined using a UV detector with wavelengths set at 254 and 280 nm. Samples were prepared by dissolving the crude material in the smallest amount of compatible solvent. Silica gel (mesh size 70-230) was then added to adsorb the material. Excess solvent was vacuum evaporated and the sample was loaded into a RediSep® Rf 25 g sample cartridge (catalog # 69-3873-240).
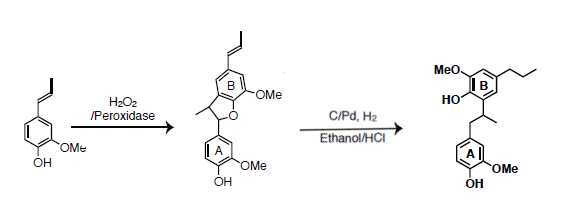
Licarin a can be purified by recrystallization from methanol or by flash chromatography. Column used was a RediSep® Silver 80 g silica gel flash column (catalog # 69-2203-380). Solvent system was hexane (Solvent A) and ethyl acetate (Solvent B). Licarin a was separated from impurities using a ratio of 1:4 ethyl acetate:hexane.
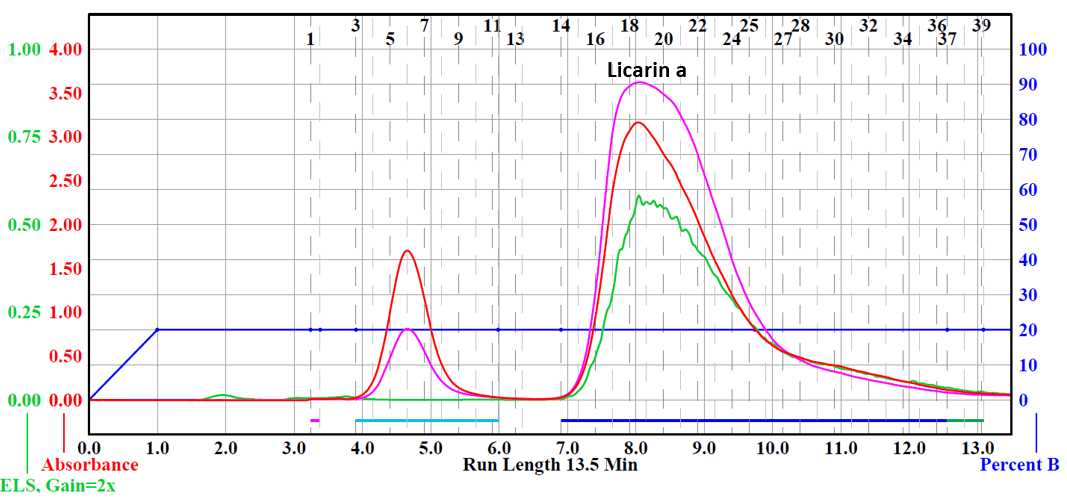
2-(1-(4-hydroxy-3-methoxyphenyl)propan-2-yl)-6-methoxy-4-propylphenol was purified via flash chromatography. Column used was a RediSep® Silver 40 g silica gel flash column (catalog # 69-2203-340). Solvent system was hexane (Solvent A) and ethyl acetate (Solvent B). Material was separated from impurities using a ratio of 15% ethyl acetate and 75% hexane.
NMR Spectroscopy
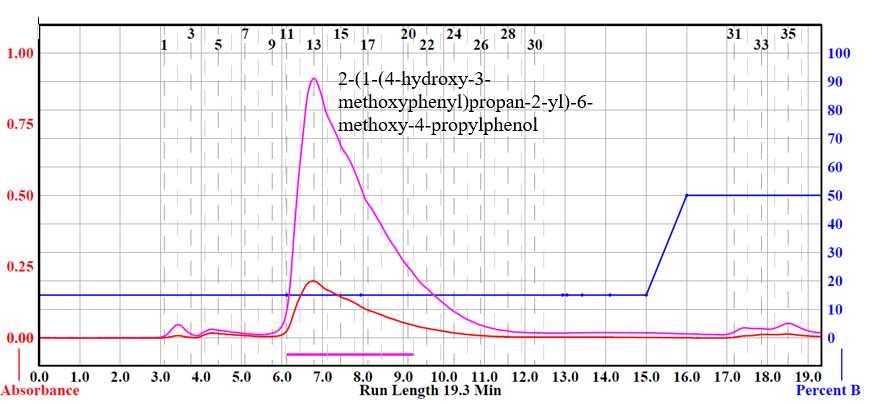
Nuclear magnetic resonance (NMR) spectra are acquired in a suitable deuterated NMR solvent at 25°C on a Bruker AVANCE 400 MHz spectrometer equipped with a 5 mm BBO probe. Chemical shifts (δ) are reported in ppm. 1H-NMR spectra are recorded with a relaxation delay of 1.0 s and an acquisition time of 4.09 s. The acquisition parameters for 13C-NMR include a 90˚ pulse width, a relaxation delay of 1.0 s, and an acquisition time of 1.36 s. Tetramethylsilane is used as a reference.
¹H NMR (400 MHz, d6-acetone): δ 7.67 (s, 1H, ArOH), 7.11-6.84 (m, 5H, aromatic region), 6.40 (dd, J =14.2, 1.6 Hz, 1H, Bα), 6.18 (dq, J =9.1, 6.6 Hz, 1H, Bβ), 5.10 (d, J=9.3 Hz, 1H, Aα), 3.86 (s, 3H, OMe), 3.85 (s, 3H, OMe), 3.45 (dq, J =6.8, 2.3 Hz, 1H, Aβ), 1.84 (dd, J =4.9, 1.6 Hz, 3H, Bγ), 1.38 (d, J= 6.8 Hz, 3H, Aγ).
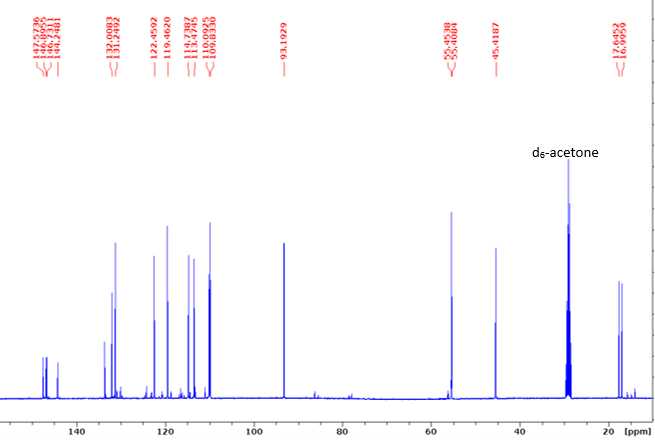
¹³C NMR (100 MHz, d6-acetone): δ 147.6 (A3), 146.9 (B3), 146.7 (A4), 144.2 (B4), 133.2 (B1), 132.0 (B5), 131.2 (A1), 130.9 (Bα), 122.4 (Bβ), 119.5 (A6), 114.7 (B6), 113.5 (A5), 110.1 (B2), 109.8 (A2), 93.2 (Aα), 55.5 (OMe), 55.4 (OMe), 45.4 (Aβ), 17.6 (Bγ), 16.9 (Aγ).
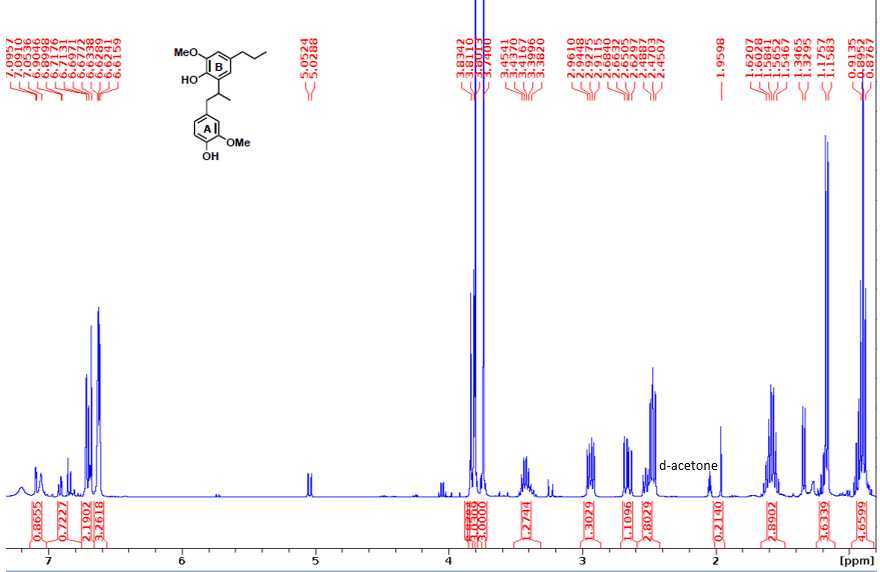
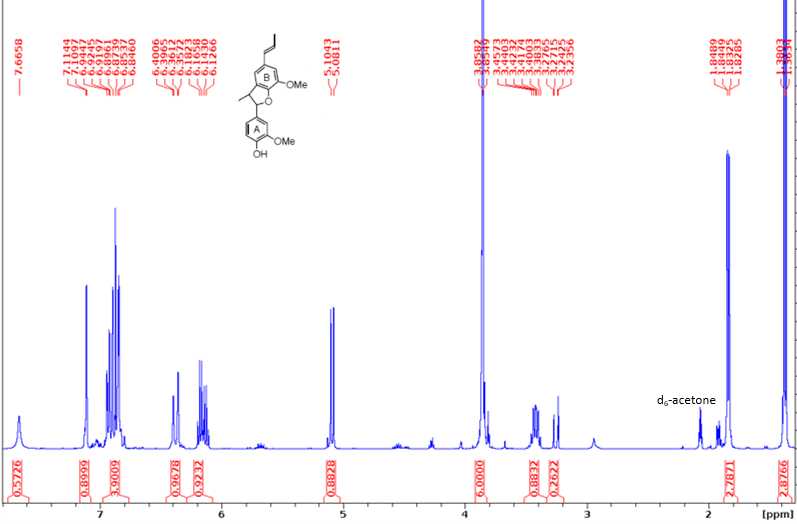
1H NMR (400 MHz, d6-acetone): δ 6.72 (d, J = 1.8 Hz, 1H, A2), 6.69 (d, J = 7.9 Hz, 1H, A5), 6.63 (d, J = 1.9 Hz, 1H, B2), 6.62 (dd, J = 6.8, 1.2 Hz, 1H, A6), 6.61 (d, J = 1.2 Hz, 1H, B6), 3.81 (s, 3H, B3-OMe), 3.74 (s, 3H, A3-OMe), 3.45 (sex, J = 6.8 Hz, 1H, Aβ), 2.96 (dd, J = 6.6 Hz, 1H, Aα1), 2.68 (dd, J = 8.3, 5.1 Hz, 1H, Aα2), 2.48 (t, J = 7.4 Hz, 2H, Bα), 1.62 (sex, J = 7.2 Hz, 2H, Bβ), 1.17 (d, J = 6.9 Hz, 3H, Aγ), 0.91 (t, J = 7.3 Hz, 3H, Bγ).
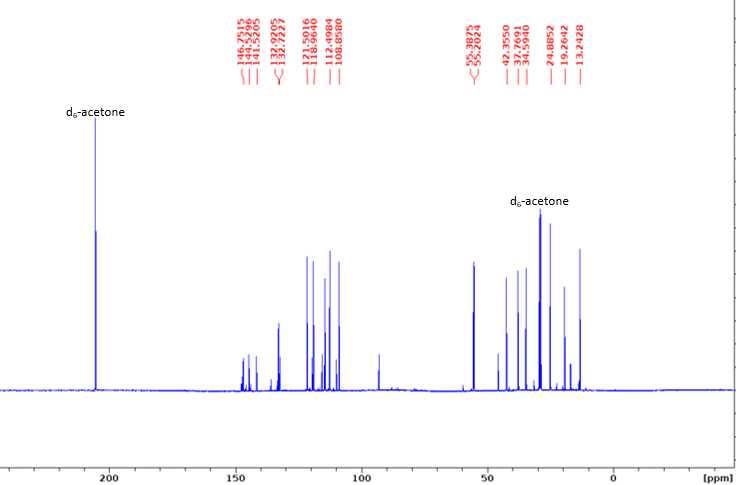
13C NMR (100 MHz, d6-acetone): δ 146.75 (A3), 146.31 (B3), 144.53 (A4), 141.52 (B4), 132.92 (B1), 132.72 (A1), 132.04 (B5), 121.50 (A6), 118.96 (B6), 114.22 (A5), 112.50 (A2), 108.86 (B2), 55.39 (B-OMe), 55.20 (A-OMe), 42.36 (Aα), 37.77 (Bα), 34.59 (Aβ), 24.89 (Bβ), 19.26 (Aγ), 13.24 (Bγ).
[4]