Mouse Water Restriction
Avalon Amaya, Jackie Swapp
Abstract
This protocol describes a semi-customized workflow to perform water restriction with mice designated for future experiments.
Water restriction is a common method used to increase engagement and motivation of mice performing behavioral experiments that use water as a reward.
Water restriction timeline summary: Mice should have three daily weights collected, which are averaged to determine their baseline weight. The target weight will be a percentage (desired) of their baseline weight. The first day of water restriction begins on the last day of the mouses weight collection and is given 1.0mL of water. The mouse should be weighed and provided at least 1.0mL of water daily while under water restriction. All calculations should be individually determined for each mouse.
Mice under water restriction should be closely monitored for health concerns due to the effects of dehydration. All mice should receive 1.0mL of water daily. Follow IACUC and veterinary requirements for water restricting mice.
Before start
Steps
Calculating Baseline Weight and Restriction
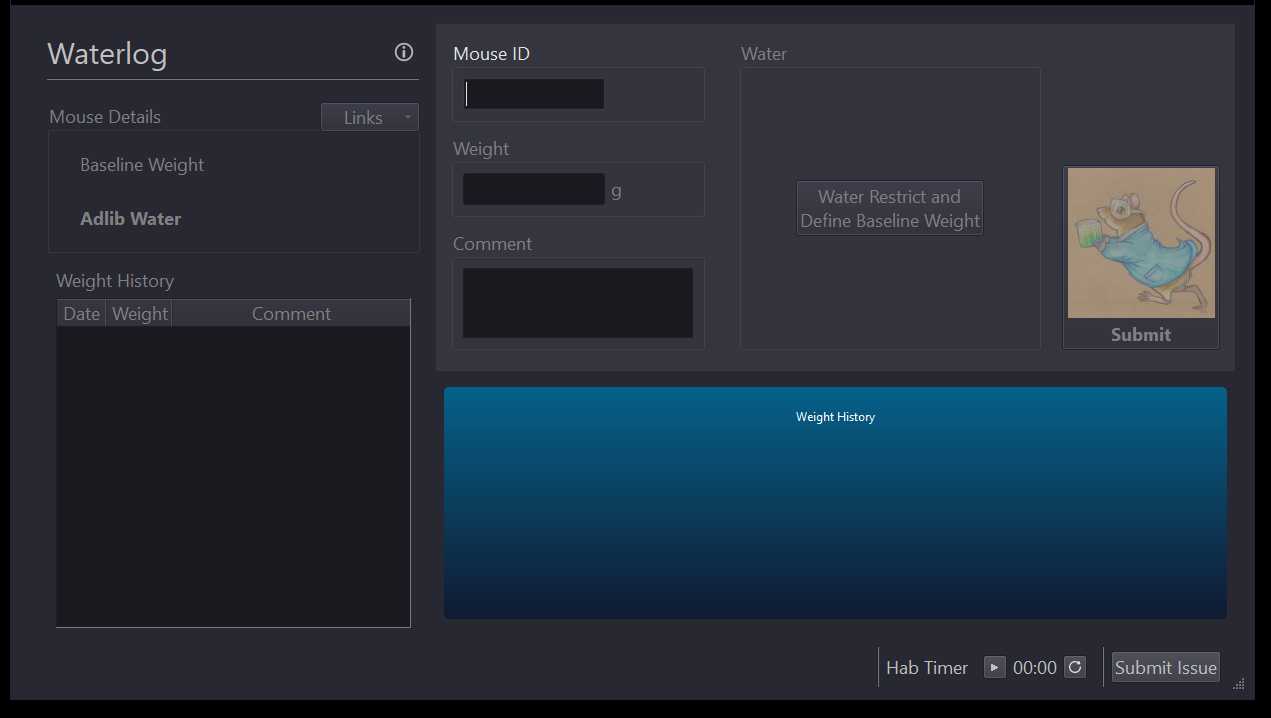
Open Waterlog via desktop application.
Enter mouse ID in mouse ID text box and press enter on your keyboard.
Retrieve mouse from the animal holding room (AHR).
If transporting mice under reverse light cycle, utilize a cart cover within non-red light rooms.
Ensure that mouse has correct enrichment in their cage based on requirements of the experimental design and genotype (i.e. tube, wheel, chewing block, etc.).
Collect and record daily weight of mouse on ad-lib water for at least 3 days.
Scoop/cup mouse out of cage.
Check mouse for correct ID (ear notches and/or tattoo). Check mouse for any other wellness concerns (headframe, prolapse, wounds, etc.).
Place mouse in weigh container and place on scale. Note the weight.
Return mouse back to cage.
Clean weigh container with 70% Ethanol spray after each use. This is to prevent any additional stress on mice caused by previous mice excrement.
Record mouse weight in weight textbox of Waterlog. Click submit button.
Place mouse on non-water restriction rack with water spouts and/or attach a filled water bottle to cage for the first two days.
Toggle water spout prior to placing mouse on rack to confirm that water spout is delivering water.
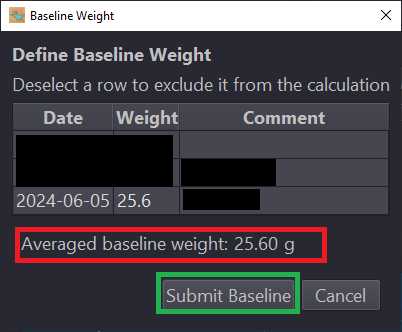
On the third day use Waterlog to calculate the average of your recorded weights to obtain the mouse's baseline weight.
Open Waterlog and enter LIMS User ID.
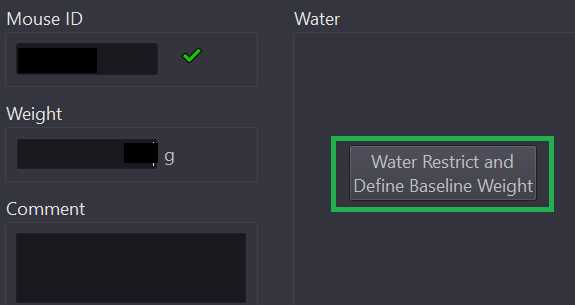
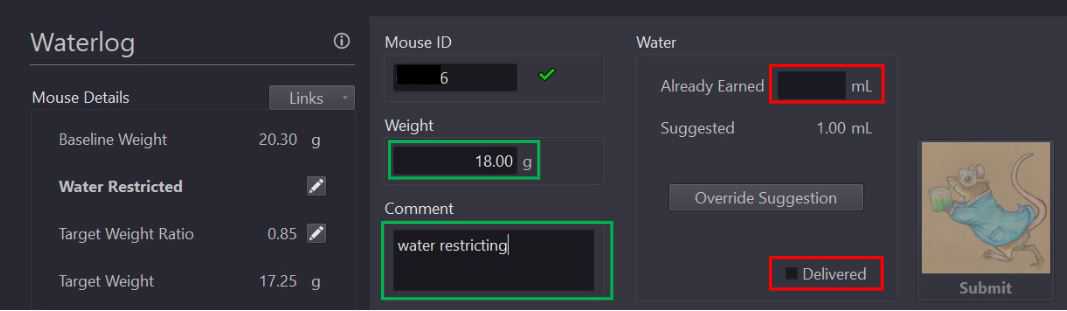
Enter mouse weight into the current weight text box. Enter any behaviorally relevant information (behavior program title, animal performance notes, hardware/software issues, etc.) in the comment text box.
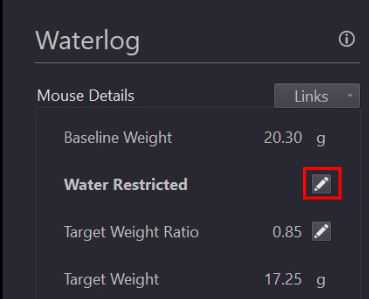
Click the "Water Restrict and Define Baseline Weight" button (outlined in green above).
Confirm mouse ID, sex, pedigree and protocol between mouse horizontal cage card, "links" dropdown menu in Waterlog, and personal schedule.
Confirm baseline weight, target ratio, and target weight calculations in Mouse Details section of Waterlog page.
Within the AHR, locate the hose for the external cage rack water supply. This is typically behind the cage racks.
If Waterlog is not accessible, manually calculate the mouse's baseline and target weight.
Calculate the average weight of the mouse with a minimum of three recently collected weights. This is your baseline weight.
Multiply your baseline weight by the desired water restriction percentage (as specified by your IACUC protocol).
For example:
-
Mouse has a baseline weight of 25.00 grams.
-
Desired water restriction at 85% of weight.
-
25.00 grams x 0.85 = 21.25 grams.
-
Maintain mouse at target weight of 21.25 grams.
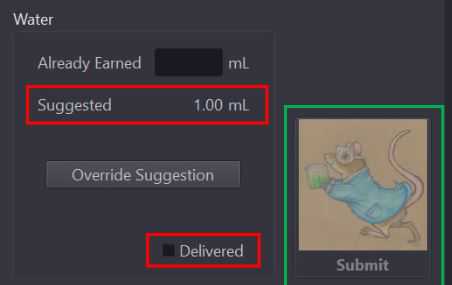
Using the water bottle with syringe, pull up 1.0mL of water and provide to mouse via water dish in cage.
Select the "delivered" checkbox and click the "submit" button to the right to submit the mouses watering information for the day.
Record necessary information on physical cards on mouse cage.
Return mouse to water restriction rack (racks with detached water valves) in AHR.
Maintaining a Mouse on Water Restriction
For each day the mouse is on water restriction, the mouse will need to be given their calculated water atollment each day.
Retrieve mouse cage from AHR. Confirm mouse ID on all cage cards.
Open Waterlog and access mouse record by repeating
Confirm mouse ID, sex, pedigree and protocol between mouse horizontal cage card, "links" dropdown menu, and personal schedule.
Confirm baseline weight, target ratio, and target weight calculations in Mouse Details section of Waterlog

Weigh mouse for each day on water restriction to maintain mouse at target weight.
Scoop/cup mouse out of cage.
Check mouse for correct ID (ear notches and/or tattoo). Check mouse for any other wellness concerns (headframe, prolapse, wounds, etc.).
Place mouse in weigh container and place on scale. Note the weight.
Return mouse back to cage.
Clean weigh container with 70% Ethanol spray after each use. This is to prevent any additional stress on mice caused by previous mice excrement.
Record mouse weight in weight textbox of Waterlog.
Enter water amount received prior to weighing in "already earned" textbox.
Provide mouse with [now] calculated water allotment as written in "suggested" textbox.
If Waterlog is not accessible, manually calculate the mouse's water allotment.
Water allotment calculation (no previous water rewards):
-
Mouse weight is 20.00 grams.
-
Baseline weight is 21.25 grams.
-
21.25 grams - 20.00 grams = 1.25 grams.
-
1.0mL = 1.0 grams.
-
Provide mouse 1.25 mL of water.
Water allotment calculation (previous water rewards):
-
Mouse weight is 20.00 grams.
-
Baseline weight is 21.25 grams.
-
Mouse received 0.75mL of water reward.
-
21.25 grams - 20.00 grams = 1.25 grams.
-
1.0mL = 1.0 grams.
-
1.25 mL - 0.75mL = 0.5mL.
-
Provide mouse 0.5 mL of water.
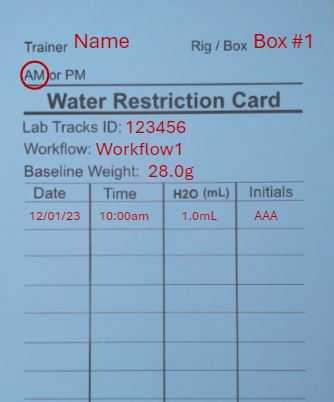
Record mouse watering information on vertical water restriction card including:
-
Date
-
Time
-
Total water provided
-
Initials
Return mouse to water restriction rack (racks with detached water valves) in AHR.
Taking a Mouse off Water Restriction
Repeat section "Maintaining a Mouse on Water Restriction". Specifically .
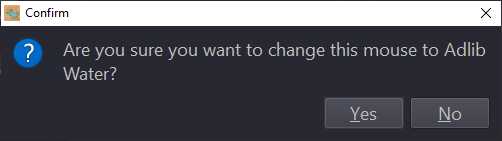
Remove mouse from water restriction in Waterlog.
Confirm that mouse is to be removed from water restriction.
Allow the mouse to drink it's alloted water amount, and then remove water dish from cage.
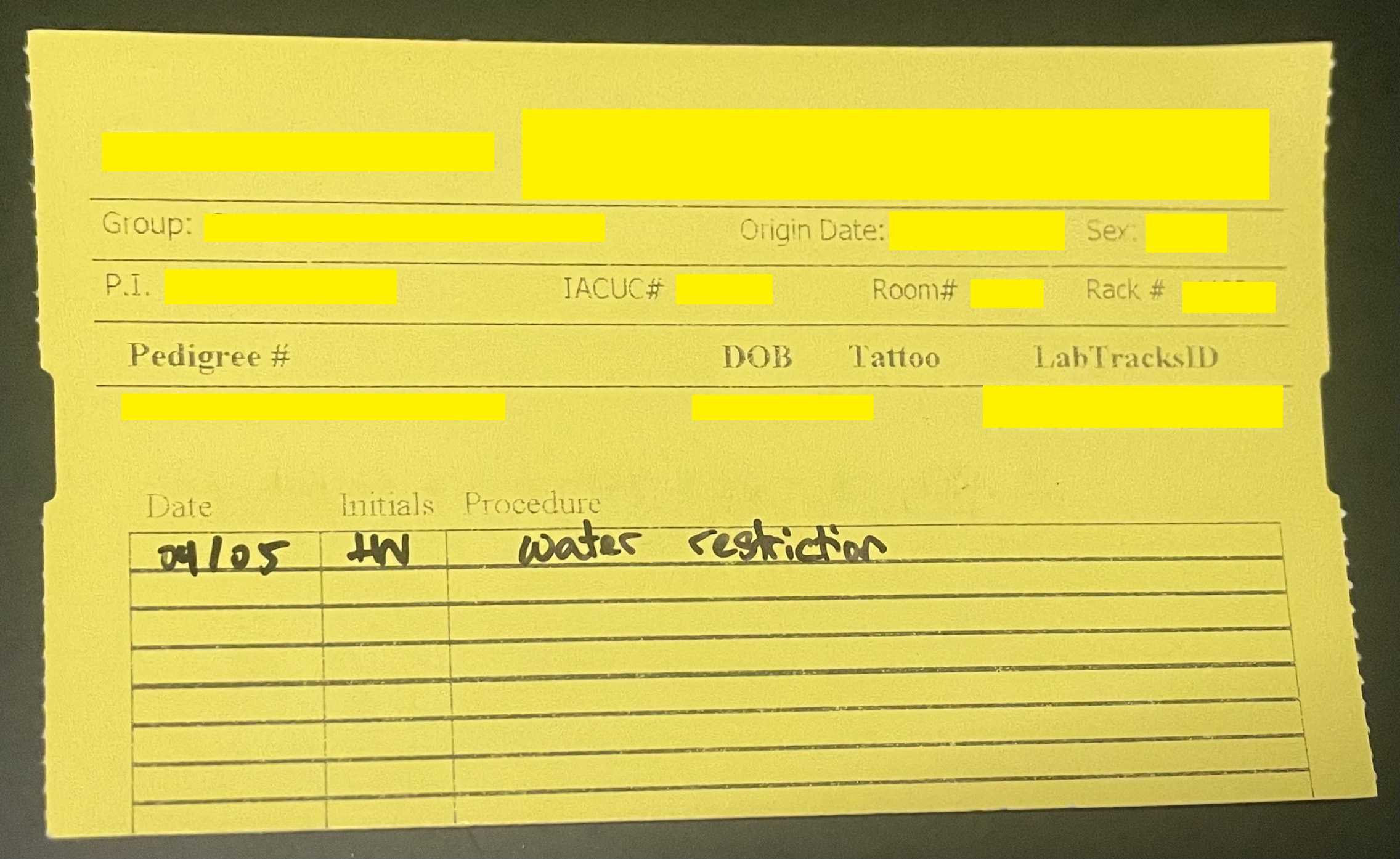
Record on horizontal animal cage card that animal has been placed on adlib water. Include the date.
Retrieve the "placed back on adlib" cage flag and place on cage over cage cards. Write the date.
Place mouse on non-water restriction rack with water spouts and/or attach a filled water bottle to cage.
Toggle water spout prior to placing mouse on rack to confirm that water spout is delivering water.
Place used water dish in dirty bin of AHR.