In vitro co-culture system using a fiber-supported liquid approach
Alejandro Calle, Jeffrey Adelberg, Guido Schnabel, Jacqueline Naylor-Adelberg, Jhulia Gelain, Yeter Karakoc, Jared Weaver, Christopher Saski, Ksenija Gasic
Abstract
In vitro co-culture techniques that allow the growth of plants and pathogens under controlled environmental conditions are being used to re-create host plant infection. These approaches reduce infection times, promote reproducibility, and enable a rapid evaluation of plant-pathogen interactions. As a result, these systems have become essential in breeding programs aimed at developing plant resistance to diseases. In this study, we developed and validated an in vitro co-culture system to investigate the Armillaria root rot (ARR) affecting Prunus spp. This disease, caused by fungi Armillaria spp. and Desarmillaria caespitosa , poses
a severe threat to the stone and nut fruit industry due to the susceptibility of most commercial rootstocks to infection and the lack of effective management options for its control. The system consists of a fiber-supported liquid approach in sterile plastic vessels that allows a fast and reproducible fungal infection under controlled environmental conditions. The floor of the vessels was covered with a polyester-fiber matte and a germination paper that served as an interface between the mycelia and the plant roots. The vessels were subjected to inoculation with Armillaria mellea a and D. caespitosa a, and three Prunus genotypes (‘Guardia®‱’, ‘MP-29’, and Prunus cerasifera a ‘14-4’) were co-cultured with both fungi. Disease progression and plant and fungal biomass were monitored during co-culture. The presented in vitro co-culture approach facilitates the concurrent growth of Armillaria/Desarmillaria spp. and Prunus s spp., excluding most of the limitations associated with greenhouses and field experiments. This system provides consistent and reproducible conditions for investigating a prominent plant disease affecting Prunus spp.
Steps
Establishment of Plant Cultures
Establishment of plant cultures from dormant shoots
Collect dormant shoots, cut them (3 cm in length),and cleanse them by submerging in 70% ethanol for 1 minute, followed by rinsing with sterile deionized water. Then, immerse the shoots in a 10% bleach solution for 10 minutes, and rinse them twice with deionized water (Figure 1).
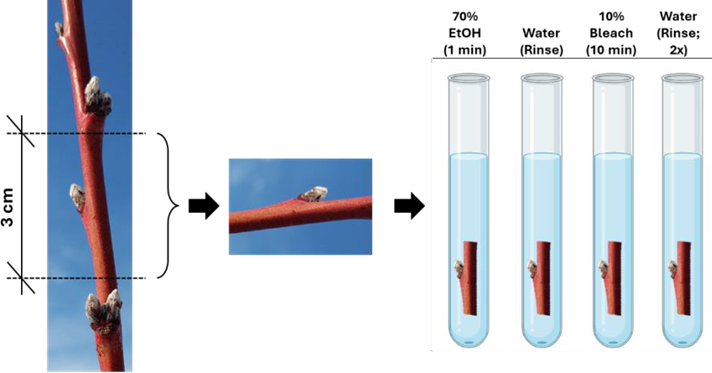
Peel the vegetative shoot buds and transfer them into culture tubes containing 20 mL of Murashige and Skoog agar media. Place the shoot vertically ensuring that the bud is 1 cm above the agar media.
Establishment of plant cultures from seeds
Clean fruit exocarps with 20% bleach for 10 minutes, followed by a 10-minute immersion in 70% ethanol.
Extract seeds within a laminar flow hood and transfer them aseptically into culture tubes containing Woody Plant Medium. Allow them to undergo stratification for ten weeks in darkness at 4 ºC.
Maintenance of stock plants
Sustain stock plants in Magenta GA-7 vessels by transferring shoot tips every five weeks onto a fresh medium.
Maintain vessels under a photosynthetic photon flux density of 20 μmol/s/m2, with a 16-hour photoperiod at 24°C.
Optional: When the When the presence of hyper multiplication, an occasional resting cycle with 16 μM indole-3-acetic acid (resting media) is recommended.
Fungi Preservation
Seal plates with parafilm and maintain in the dark at 20 ºC.
Refresh every 14 days by transferring mycelial plugs to fresh MEA plates to ensure active fungal growth.
Inoculum preparation
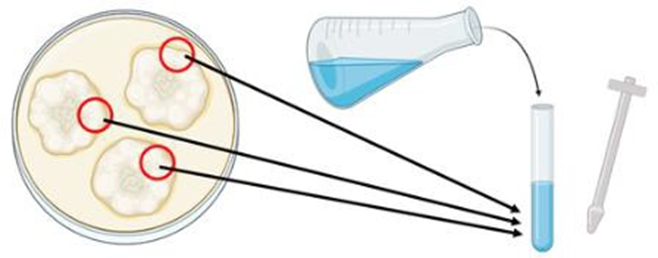
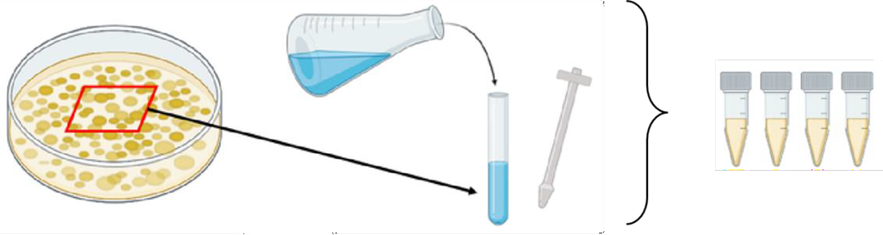
Extract three ten-millimeter-diameter plugs from the edge of two-week-old colonies
Place a sterile ultra-clear porous cellophane sheet on top of a Petri dish containing malt extract agar media and pour 600 µL of homogenate
Spread homogenate uniformly over the entire plate using a cell spreader and incubate the plate in the dark for 14 days.
Co-culture (plant-fungi) establishment
Autoclave (121 ºC for 20 min) rectangular plastic vessels (110 × 297 mm; Southern Sun
BioSystems) and after cooling down to room temperature, set the fiber-supported
paper on the floor of each vessel (Figure 6).


Add 175 mL of plant growth regulator-free liquid ‘New Prunus Medium’ to each vessel.
Place vessels on a rocker's arm with an articulated shelf that providesone swing every 15 min.
Use another set of rectangular Southern Sun BioSystems vessels containing 175 mL of ‘New Prunus Medium’, fiber matte, and germination paper and add 1 mL of the mycelium suspension for inoculum.
Seal the vessels with PVC film and place them on an automatic rocker arm at 5 rpm under μM/m2/s LED light 2 red 1 blue and 16 h/day photoperiod at 24 ºC.
After ten and seventeen days of inoculation with A. mellea and D. caespitosa , respectively, transfer the in vitro rooted plants from the liquid media to the inoculated vessels.
Add 60 mL of ‘New Prunus Medium’ without any plant growth regulator just before plant transferring.
Seal the vessels with PVC film and place them on an automatic rocker arm at 5 rpm under μM/m2/s LED light 2 red 1 blue and 16 h/day photoperiod at 24 ºC (Figure 8).

Collect tissues when needed.