Extraction and LC-MS/MS analysis of four steroids from mouse plasma and bone marrow
Natalie ZM Homer, Andrea Lovdel, Scott G Denham, William P Cawthorn
steroid
steroid analysis
quantitative steroid measurement
LC-MS/MS
corticosterone
11-dehydrocorticosterone
testosterone
progesterone
supported liquid extraction
mouse plasma
mouse bone marrow
bone marrow
mouse
tissue specific
tandem mass spectrometry
steroid mass spectrometry
Abstract
Steroid hormones, including progesterone, testosterone and corticosterone, play a critical role in
growth, development, reproductive function and sexual differentiation. Steroid production is controlled in the adrenals via the hypothalamic-pituitary-adrenal axis and in the gonads by the hypothalamic-pituitary-gonadal axis, but steroids are also produced and metabolised in peripheral tissues such as adipose tissue. Circulating steroids (blood levels) do not always reflect local steroid tissue levels. In studies of caloric restriction there are observed changes in steroids and there is interest in the role that bone marrow adipose tissue play11. Thus, to understand mechanisms of caloric restriction it is valuable to measure tissue levels of steroids as well as circulating levels of steroids.
Immunoassays can be used to measure steroids but they lack specificity, limited to one or two steroids, and suffer from cross-reactivity at low concentrations. Tandem mass spectrometry methods coupled with chromatographic separation are considered the gold standard analytical technique for steroid analysis, such as LC-MS/MS, with the added benefit of enabling simultaneous analysis of multiple steroids.
Here we have improved upon existing murine steroid liquid chromatography tandem mass spectrometry (LC-MS/MS) methods2 for the quantitation of four steroids in small samples to investigate the role of steroids in caloric restriction - specifically corticosterone, 11-dehydrocorticosterone, testosterone and progesterone - in bone marrow and plasma. Bone marrow is homogenised and then homogenate samples and plasma samples (~50 µL) were extracted by automated 96-well supported liquid extraction (SLE), using dichloromethane and isopropanol as an organic solvent, carried out on a Biotage Extrahera automated sample handler.
Extracted steroids were separated on a Shimadzu Nexera uHPLC with gradient elution on a Kinetex C18 column (150 x 3 mm; 2.6 µm) and a mobile phase of methanol and water (0.1% formic acid). The run time was 16 minutes, followed by mass spectral analysis on an AB Sciex 6500+ tandem quadrupole mass spectrometer operated in positive ionisation mode.
This automated SLE-LC-MS/MS method has been used to analyse 4 steroids - corticosterone, progesterone and testosterone - in mouse plasma and bone marrow. Validation demonstrates that this method is sensitive, specific, and suitable for steroid measurement in mouse bone marrow (4-8 bones) and a low volume of mouse plasma (50 µL), enabling investigation into tissue specific steroid levels and corresponding assessment of circulating steroid levels.
Before start
Ensure all consumables are in stock and all compounds and reagents are freshly prepared
Attachments
Steps
Bone Marrow Collection and Homogenisation
Prepare 0.5 mL Eppendorf microtubes for all bone marrow samples by removing the bottom of a 0.5 mL microtube (Eppendorf) with a razor blade
Batch sizes of 20-40 bone marrow samples are most manageable. Isolate bone marrow (BM) from frozen (or fresh) tibia by doing the following:
Remove tibiae from freezer and transfer frozen tibiae from dry ice onto wet ice prior to cutting.
Use a razor blade to cut off the proximal and distal ends of each tibia
Place each cut tibia into a cut-off 0.5 mL microtube, to keep the bone upright, and add this tibia in a 0.5 mL microtube into a 2 mL microtube.
Centrifuge the 2 mL cap-lock microtube and its contents in a microcentrifuge for8000rcf,4°C. The BM pellet will collect at the base of the 2 mL microtube
Record the mass of each BM pellet, using an analytical balance, prior to homogenisation. Multiple BM pellets can be combined at this point. (e.g. 2, 4 , 6 or 8 BM pellets can be homogenised together).
Add 500µL to the bone marrow in the 2 mL microtube
Add a 5 mm stainless steel metal bead into a 2 mL microtube, cap securely and homogenise in the Qiagen bead mill homogeniser at 30 Hz for 0h 0m 30s
Remove and transfer the homogenisation tube containing the homogenate to the freezer for 1h 0m 0s at -20°C
Remove the homogenate tube from the freezer, centrifuge for 0h 5m 0s at 16,100 rcf
Transfer the homogenate to a clean, labelled glass vial using a glass pipette
Add another aliquot of 500µL to the bone marrow pellet, vortex and centrifuge for 0h 5m 0s at 16,100 rcf
Transfer the supernatant from the homogenisation tube and add it to the first supernatant in the glass tube.
Reduced the supernatant to dryness under nitrogen at 40°C
Resuspend the dried down homogenate supernatant ofSample by adding 200µL prior to preparation of calibration standards (4), working internal standard (5) and then supported liquid extraction (See point 7) of steroids
Preparation of mouse plasma for extraction
Remove mouse plasma samples from the freezer and defrost on ice. Label up glass culture tubes with mouse sample IDs and aliquot 50µL of each Sample into labelled glass tubes, alongside bone marrow. Record exact volume of plasma aliquoted in plate map.
Preparation of calibration standard solutions
Prepare 100 µg/mL stock solutions of each steroid - corticosterone (B), 11-dehydrocorticosterone (A), testosterone (T) and progesterone (P4) in 1.75 m
Prepare a mixed stock of the 4 steroids - B, A, T, P4 - by using 100 µg/mL stock solutions. Do this by adding 50 µL x 100 µg/mL B, 50 µL x 100 µg/mL A, 50 µL x 100 µg/mL T and 50 µL x 100 µg/mL P4 + 800 µL methanol to give a 5 µg/mL stock .
Dilute the 5 µg/mL stock Mixed STOCK by 1:10 dilution (100 µL x 5 µg/mL + 900 µL methanol ) to give 500 ng/mL stock
Dilute the 500 ng/mL mixed STOCK by 1:10 dilution (100 µL x 500 ng/mL + 900 µL methanol ) to give 50 ng/mL stock
Dilute the 50 ng/mL mixed STOCK by 1:10 dilution (100 µL x 5 µg/mL + 900 µL methanol ) to give 5 ng/mL stock
Dilute the 5 ng/mL Mixed STOCK by 1:10 dilution (100 µL x 5 µg/mL + 900 µL methanol ) to give 500 pg/mL stock
Dilute the 500 pg/mL Mixed STOCK by 1:10 dilution (100µL x 5 µg/mL + 900µLmethanol ) to give 50 pg/mL stock
Preparation of internal standard solution
Prepare 100 µg/mL solutions of each isotopically labelled internal standard (d8-corticosterone, 13C3-testosterone and d9-progesterone) in methanol.
Prepare a mixed 5 µg/mL Internal Standard mix stock solution of the three isotopically labelled steroids by adding 25µL x 100 µg/mL d8-corticosterone, 25µL x 100 µg/mL 13C3-testosterone and25µL x 100 µg/mL d9-progesterone to425µL methanol.
Prepare a 5 ng/mL Working Internal Standard solution by taking 10 µL x 5 µg/mL Int Std Mix + 1990 µL methanol.
Preparation of calibration standards
Prepare calibration standards directly into labelled glass culture tubes using the following table for volumes of each stock concentration, into a final volume of 200 µL water.
| A | B | C | D |
|---|---|---|---|
| Standard name | Amount (ng) | STD Mix Vol (uL) | Vol water (uL) |
| 0 STD | 0 | 0 | 200 |
| 0.00250 STD | 0.00250 | 5 uL x 500 pg/mL | 195 |
| 0.00500 STD | 0.00500 | 10 uL x 500 pg/mL | 190 |
| 0.01000 STD | 0.0100 | 20 uL x 500 pg/mL | 180 |
| 0.0250 STD | 0.0250 | 5 uL x 5 ng/mL | 195 |
| 0.0500 STD | 0.0500 | 10 uL x 5 ng/mL | 190 |
| 0.100 STD | 0.100 | 20 uL x 5 ng/mL | 180 |
| 0.250 STD | 0.250 | 5 uL x 50 ng/mL | 195 |
| 0.500 STD | 0.500 | 10 uL x 50 ng/mL | 190 |
| 1.00 STD | 1.00 | 20 uL x 50 ng/mL | 180 |
| 2.50 STD | 2.50 | 5 uL x 500 ng/mL | 195 |
| 5.00 STD | 5.00 | 10 uL x 500 ng/mL | 190 |
| 10.0 STD | 10.0 | 20 uL x 500 ng/mL | 180 |
Calibration standard preparation table
Supported liquid extraction of steroids from calibration standards and samples
Transfer 200 µL of prepared calibration standards, bone marrow homogenate and mouse plasma from glass culture tubes into a 2 mL deep well 96-well collection plate (Biotage), prepared in Microsoft Excel template (see Files) and following a plate map design as below -
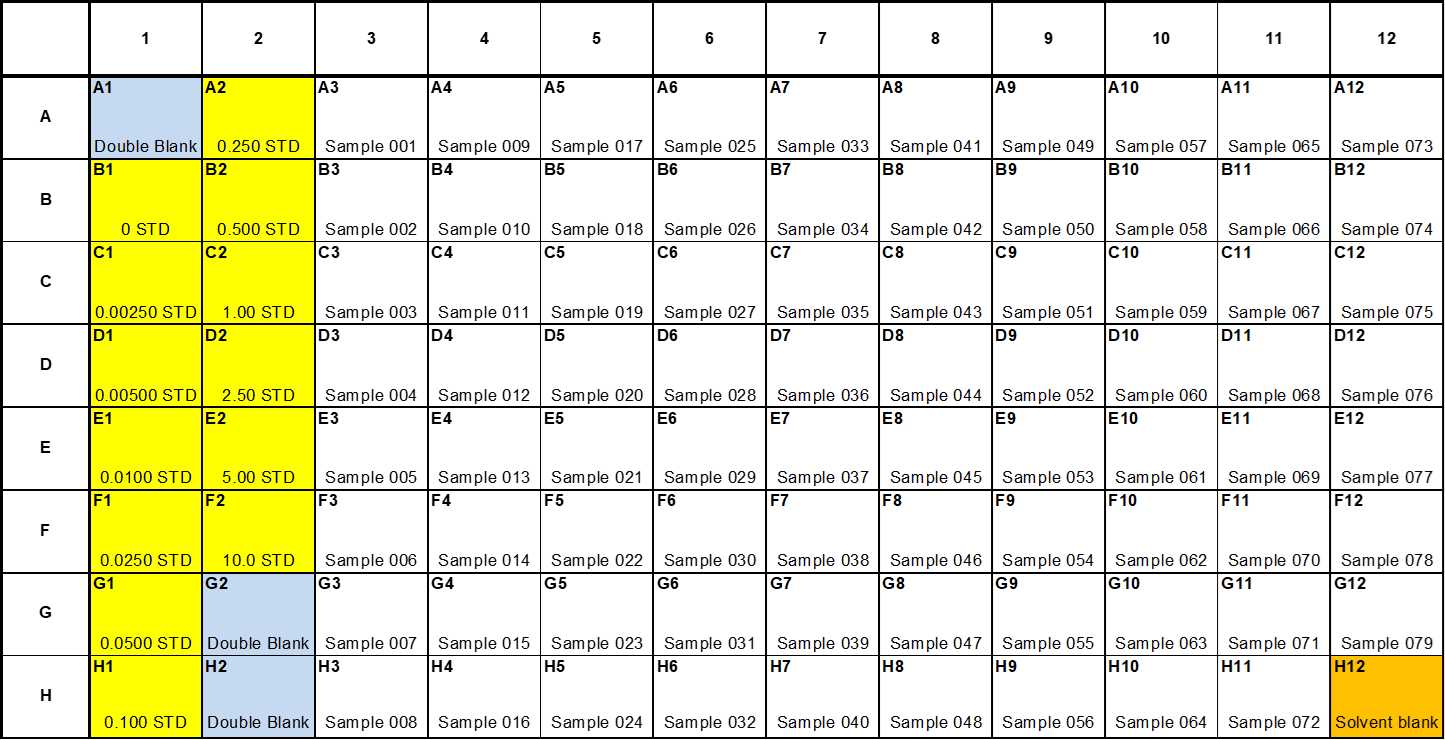
Using a multi-step pipette enrich the plate containing calibration standards with IS by adding 20µL x 5 ng/mL Working Internal Standard into each calibration standard, including 0 std and each sample (bone homogenate and mouse plasma), except for the Double blank and solvent blank.
Using the Extrahera liquid handling robot, set up with an SLE400 extraction plate and a deep well extraction plate, aliquot 200 µL 0.5Molarity (M)ammonium hydroxide in water into each well of the 96-well plate.
Transfer 400µL of liquid from each well (containing sample and the diluent, 0.5Molarity (M)ammonium hydroxide) into a 400 µL volume Supported Liquid Extraction plate (SLE400), pre-placed into the deck on the Extrahera, with a deep well Waters 2 mL deep well collection plate below, pre-labelled with the batch details and date of extraction.
Allow the diluted sample to adsorb onto the SLE extraction bed for 0h 5m 0s before eluting with 600µL x 98:2 (v/v) dichloromethane/isopropanol and repeating twice more, each time collecting the eluent into the collection plate
Dry down the eluent collected into the 2 mL collection plate using the SPE Dry down for 96-well plates under nitrogen.
Resuspend in 100µL x 70:30 water/methanol, seal the plate with a zone-free plate seal and shake on ThermoShaker for 0h 5m 0s at 300rpm
Place the plate in the autosampler for LC-MS/MS or store at-20°Cuntil ready for analysis.
Steroid analysis by LC-MS/MS
Set up an acquisition batch in Analyst software using the electronic file of the calibration standards and sample list. Set to inject 10 µL per sample and use a method of chromatographic separation as described in step 16 and 17 and mass spectrometer settings as outlined in steps 18 and 19.
Set up the Shimadzu Nexera X2 liquid chromatography system and fit with a Phenomenex Krud Katcher and a Phenomenex 150 x 3 mm; 2.6 µm Kinetex C18 liquid chromatography column, using mobile phase A - water with 0.1% formic acid and mobile phase B - methanol with 0.1% formic acid at 0.5 mL/min and 40°C.
Set up chromatographic gradient as below with a run time of0h 16m 0s per sample
| A | B | C | D |
|---|---|---|---|
| Time (min) | Flow (mL/min) | A (%) | B (%) |
| Initial | 0.5 | 45 | 55 |
| 4.00 | 0.5 | 45 | 55 |
| 10.00 | 0.5 | 0 | 100 |
| 12.00 | 0.5 | 0 | 100 |
| 12.10 | 0.5 | 45 | 55 |
| 16.00 | 0.5 | 45 | 55 |
Chromatographic gradient details. A - water w/ 0.1% formic acid; B - methanol w/ 0.1% formic acid. 40oC. Kinetex C18 (150 x 3 mm; 2.6 µm)
Set up the mass spectrometer for Multiple Reaction Monitoring (MRM) method in positive mode, with electrospray ionisation as below, with divert of LC flow into the mass spectrometer set at 1 minute and 12 minutes.
| A | B |
|---|---|
| Instrument | Sciex QTrap 6500+ |
| Source, Ionisation Mode | IonDrive Turbo V Source, ESI |
| Scan Mode, Polarity | MRM, Positive |
| Resolution (Q1/Q3) | unit/unit |
| Mass range | Low mass |
| Pause Time | 5.007 ms |
| Acquisition time | 16.0 min |
| Delay time | 0 sec |
| Curtain Gas (CUR) (N2) | 30 units |
| Collision Gas (CAD) (N2) | Medium |
| IonSpray Voltage (IS) (Positive) | 4500 V |
| Temperature (TEM) | 600 °C |
| Ion Source Gas 1 (GS1) (Air) | 40 units |
| Ion Source Gas 2 (GS2) (Air) | 60 units |
| Entrance Potential (EP) (Positive) | 10 V |
| Probe position (x – axis) | 5 |
| Probe position (y – axis) | 2 |
Mass Spectrometry source settings for positive ion electrospray ionsiation on QTrap 6500+
Set up the mass spectrometer to monitor for the following MRM transitions for each steroid and isotopically labelled steroid.
| A | B | C | D | E | F | G |
|---|---|---|---|---|---|---|
| Q1 Mass (Da) | Q3 Mass (Da) | Scan time (msec) | Steroid Name | DP (V) | CE (V) | CXP (V) |
| 347.1 | 121.1 | 50 | Corticosterone 1 | 76 | 29 | 8 |
| 347.1 | 90.9 | 50 | Corticosterone 2 | 76 | 75 | 12 |
| 345.1 | 121.2 | 50 | 11-Dehydrocorticosterone 1 | 66 | 31 | 12 |
| 345.1 | 91.2 | 50 | 11-Dehydrocorticosterone 2 | 66 | 83 | 40 |
| 289.1 | 97.0 | 50 | Testosterone 1 | 101 | 29 | 12 |
| 289.1 | 109.2 | 50 | Testosterone 2 | 101 | 31 | 6 |
| 315.1 | 97.1 | 50 | Progesterone 1 | 96 | 23 | 10 |
| 315.1 | 109.1 | 50 | Progesterone 2 | 96 | 27 | 10 |
| 355.3 | 128.1 | 50 | d8B-Corticosterone 1 | 37 | 45 | 14 |
| 355.3 | 125.0 | 50 | d8B-Corticosterone 2 | 29 | 56 | 14 |
| 292.1 | 100.2 | 50 | 13C3-Testosterone | 101 | 29 | 12 |
| 324.1 | 100 | 50 | d9-Progesterone | 96 | 23 | 10 |
MRM settings for each steroid, including quantitative (1) and qualitative (2) ions for each steroid. DP - declustering potential, CE - collision energy, CXP - collision exit potential
Check the retention times of the steroids are as expected, as shown in the chromatogram below:
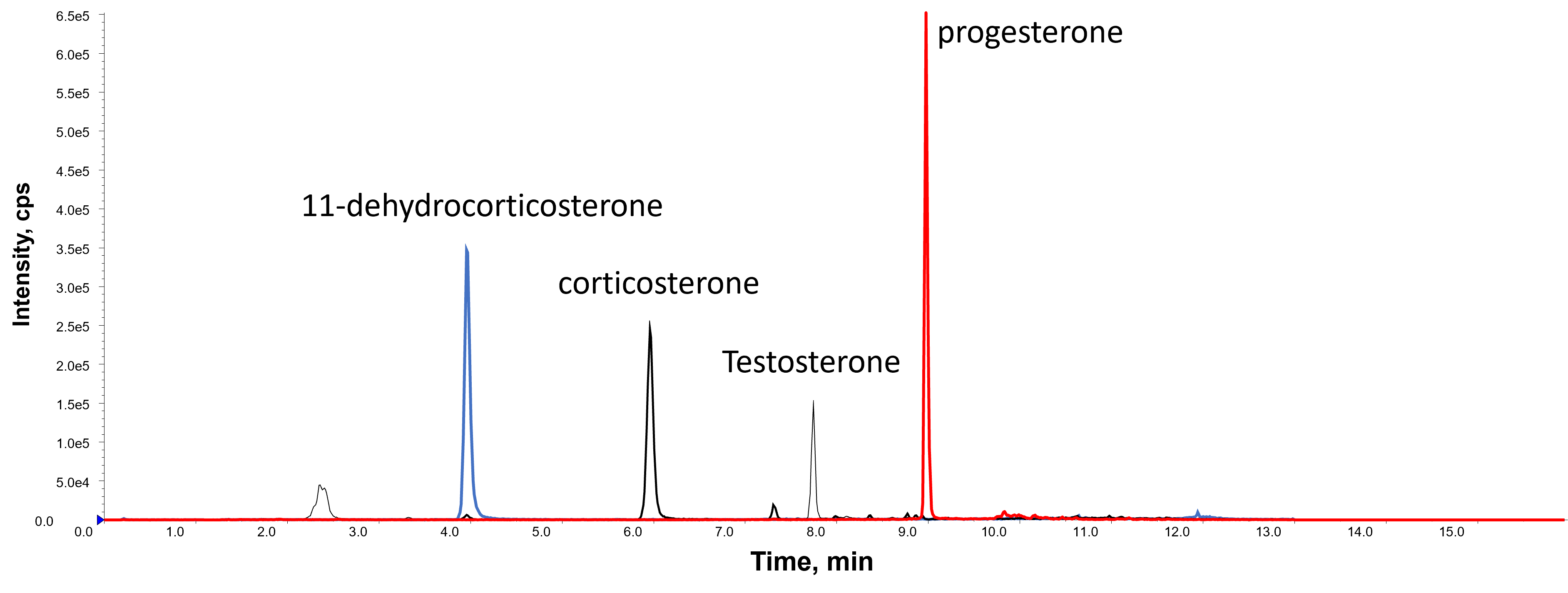
Once the chromatography has been checked and the retention times are consistent, set the batch of samples to analyse. Use MultiQuant software and excel to evaluated the LC-MS/MS data to calculate the concentration of steroids in each sample, as detailed:
Using MultiQuant and Excel software to evaluate and report multi-analyte targeted LC-MS/MS data.

