Synthetic Procedure of Dimethyl 2-methoxy-4,4'-biphenyldicarboxylate
Lisa.Stanley, Gregg T. Beckham, Caroline Hoyt
Disclaimer
This work was authored by the National Renewable Energy Laboratory, operated by Alliance for Sustainable Energy, LLC, for the U.S. Department of Energy (DOE) under Contract No. DE-AC36-08GO28308. Funding provided by U.S. Department of Energy Office of Energy Efficiency and Renewable Energy Bioenergy Technologies Office. The views expressed herein do not necessarily represent the views of the DOE or the U.S. Government.
Abstract
Lignin represents the largest source of biomass-derived aromatic chemicals and is an ideal supplement or alternative to petroleum-based feedstocks. In connection with efforts focused on oxidative lignin depolymerization, it is recognized that some of the most common products, 4-hydroxybenzoic acid ( H ), vanillic acid ( G ), and syringic acid ( S ), could serve as precursors to biaryl dicarboxylates. The parent analogue, biphenyl-4,4′-dicarboxylic acid (BPDA), has been the focus of commercial interest as a monomer for polyesters and as the core structure for nonphthalate plasticizers for poly(vinyl chloride) (PVC). Reductive coupling of phenol derivatives represents a different route to BPDA derivatives that accesses a single product regioisomer. The biomass-derived H compound provides a means to access the same BPDA analogue currently sourced from petroleum, while the G and S compounds that have methoxy substituents will afford BPDA derivatives that could have favorable properties (e.g., as a PVC plasticizer). The following protocol describes the synthetic procedure of three biaryl dicarobxylates: dimethyl 2-methoxy-4,4' biphenyldicarboxylate ( H-G ), dimethyl 2,2'-dimethoxy-4,4'-biphenyldicaroxylate ( G-G ), and biphenyl dimethyl dicarboxylate ( H-H ).
Before start
All glassware is dried in an oven set to 105ºC then cooled in a desiccator prior to use.
Steps
Synthetic Procedure
Synthesis of Methyl 4-(tosyloxy)benzoate and Methyl 3-methoxy-4-(tosyloxy)benzoate. Methyl paraben (6.6194g, 0.0435 mol), dimethylaminopyridine (0.5315g, 0.00435 mol), and triethylamine (8.805g, 0.0870 mol) were charged into a round-bottom flask. 60mL of anhydrous dichloromethane (DCM) was added to the reaction which was allowed to stir until all material dissolved. p-Toluenesuflonyl chloride (9.9531g, 0.0522 mol) was added in three parts to the mixture over two minutes. The reaction was allowed to proceed under a protective atmosphere of inert gas at room temperature for 4-5 hours.1,2 After which, the reaction was added to a separatory funnel filled with deionized (D.I.) water (80mL) and DCM (80mL). The reaction was extracted three times with DCM (60mLx3). The organic layers were combined, dried over sodium sulfate, and filtered. Solvent was removed by rotary evaporation in vacuo. The crude product was purified via flash chromatography to get methyl 4-(tosyloxy)benzoate (5.6911, 83.5%).
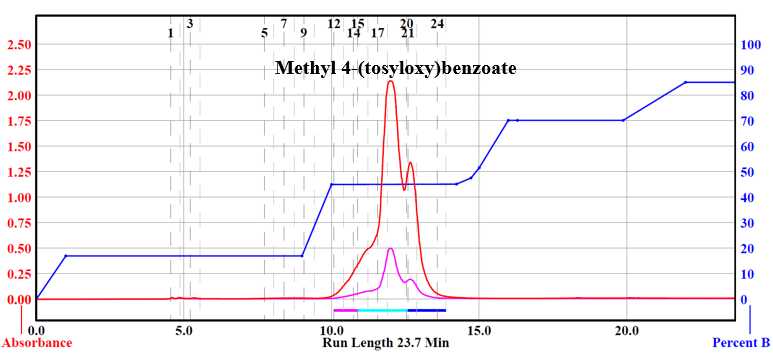
Methyl vanillate (8.000g, 0.439 mol), dimethylaminopyridine (0.5365g, 0.00439 mol), and triethylamine (8.8875g, 0.0878 mol) were charged into a round-bottom flask. 65mL of anhydrous DCM was added to the reaction which was allowed to stir until all material dissolved. p-Toluenesulfonyl chloride (10.0464g, 0.0527 mol) was added in three parts to the mixture over two minutes.1,2 The reaction was allowed to proceed under a protective atmosphere of inert gas at room temperature for 4-5 hours. After which, the reaction was added to a separatory funnel filled with D.I. water (80mL) and DCM (80mL). The reaction was extracted three times with DMC (60mLx3). The organic layers were combined, dried over sodium sulfate, and filtered. Solvent was removed by rotary evaporation in vacuo . The crude product was purified via flash chromatography to yield methyl 3-methoxy-4-(tosyloxy)benzoate (13.9814g, 93.6%).
[1]
[2]
Synthesis of Nickel Bromide Bipyridine (NiBr2bipy) catalysis. 2bipy) catalysis. Nickel (II) bromide trihydrate (10.902g, 40 mmol) was added to a round-bottom flask and dissolved in absolute ethanol (150mL). 2,2'-bipyridine (6.2476g, 40 mmol) was added to the reaction once all of the nickel (II) bromide trihydrate had dissolved. The reaction was allowed to stir at room temperature for 24 hours during which the desired product precipitates from solution [See Note 1].3 The solution was filtered to collect the product which was then dried in a vacuum dessicator to yield a light green nickel bromide bipyridine solid (10.311g, 68.8%).
[3]
Synthesis of biaryl dimer(s). Methyl 3-methoxy-4-(tosyloxy)benzoate (2.4156g, 0.00718 mol) was charged into a round-bottom flask and dissolved in 17mL anhydrous dimethylformamide (DMF). A protective atmosphere was provided and the reaction was heated to 60°C. Manganese (Mn) powder (1.1471g, 0.209 mol) was added to the reaction, then the NiBr2bipy catalyst (0.3912g, 0.001 mol), followed quickly by trifluoroacetic acid (40µL). Methyl 4-(tosyloxy)benzoate (1.000g, 0.00326 mol) in 3mL anhydrous DMF was added slowly to the reaction mixture.4 The reaction proceeded at 60ºC for 1 hour before it was acidified with 1M hydrochloric acid (20mL). Diethyl ether (50mL) was added slowly to the reaction mixture which was then extracted three times with diethyl ether (50mLx3). The organic layers were washed twice with D.I. water (50mL) and once with brine (50mL) [See Note 2]. It was then dried over sodium sulfate and concentrated in vacuo . The crude product was purified via flash chromatography to yield dimethyl 2-methoxy-4,4'-biphenyldicarboxylate (H-G dimer, 0.5506g, 58.5%), dimethyl-2,2'-dimethoxy-4,4'-biphenyldicarboxylate (G-G dimer, 0.2586g, 21.8%), and biphenyl dimethyl dicarboxylate (H-H dimer, 0.0462g, 9.4%).
[4]
Purification
Flash chromatography was performed using a Teledyne Isco Combiflash® NextGen 300+. Collected fractions were determined using a UV detector with wavelengths set at 254 and 280 nm. Samples were prepared by dissolving the crude material in the smallest amount of compatible solvent. Silica gel (mesh size 70-230) was then added to adsorb the material. Excess solvent was vacuum evaporated and the sample was loaded into a RediSep® Rf 25 g sample cartridge (catalog # 69-3873-240).
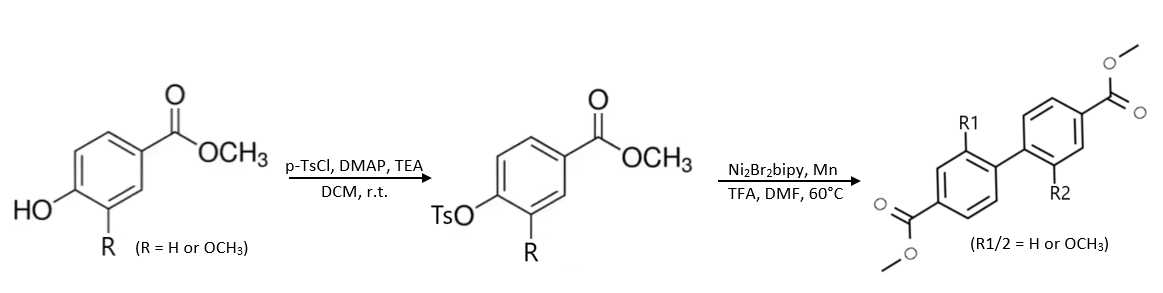
Methyl 4-(tosyloxy)benzoate was purified via flash chromatography. Column used was a RediSep® Silver 80 g silica gel flash column (catalog # 69-2203-380). Solvent system was hexane (Solvent A) and ethyl acetate (Solvent B). Methyl 4-(tosyloxy)benzoate was purified from impurities using a ratio of 45% ethyl acetate:55% hexane.
Methyl 4-(tosyloxy)benzoate was purified via flash chromatography. Column used was a RediSep® Silver 80 g silica gel flash column (catalog # 69-2203-380). Solvent system was hexane (Solvent A) and ethyl acetate (Solvent B). Methyl 4-(tosyloxy)benzoate was purified from impurities using a ratio of 45% ethyl acetate:55% hexane.
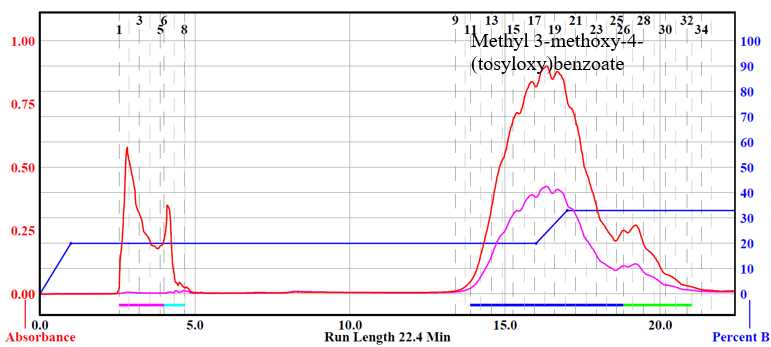
Biaryl dimers (H-G, G-G, and H-H) were purified via flash chromatography. Column used was a RediSep® Silver 40 g silica gel flash column (catalog # 69-2203-340). Solvent system was hexane (Solvent A) and ethyl acetate (Solvent B). The three dimers were purified using a ratio of 10% ethyl acetate to 90% hexane.
Nuclear Magnetic Resonance (NMR) Spectroscopy
Nuclear magnetic resonance (NMR) spectra are acquired in a suitable deuterated NMR solvent at 25°C on a Bruker AVANCE 400 MHz spectrometer equipped with a 5 mm BBO probe. Chemical shifts (δ) are reported in ppm. 1H-NMR spectra are recorded with a relaxation delay of 1.0 s and an acquisition time of 4.09 s. The acquisition parameters for 13C-NMR include a 90˚ pulse width, a relaxation delay of 1.0 s, and an acquisition time of 1.36 s. Tetramethylsilane is used as a reference.
Methyl 4-(tosyloxy)benzoate (H-OTs)
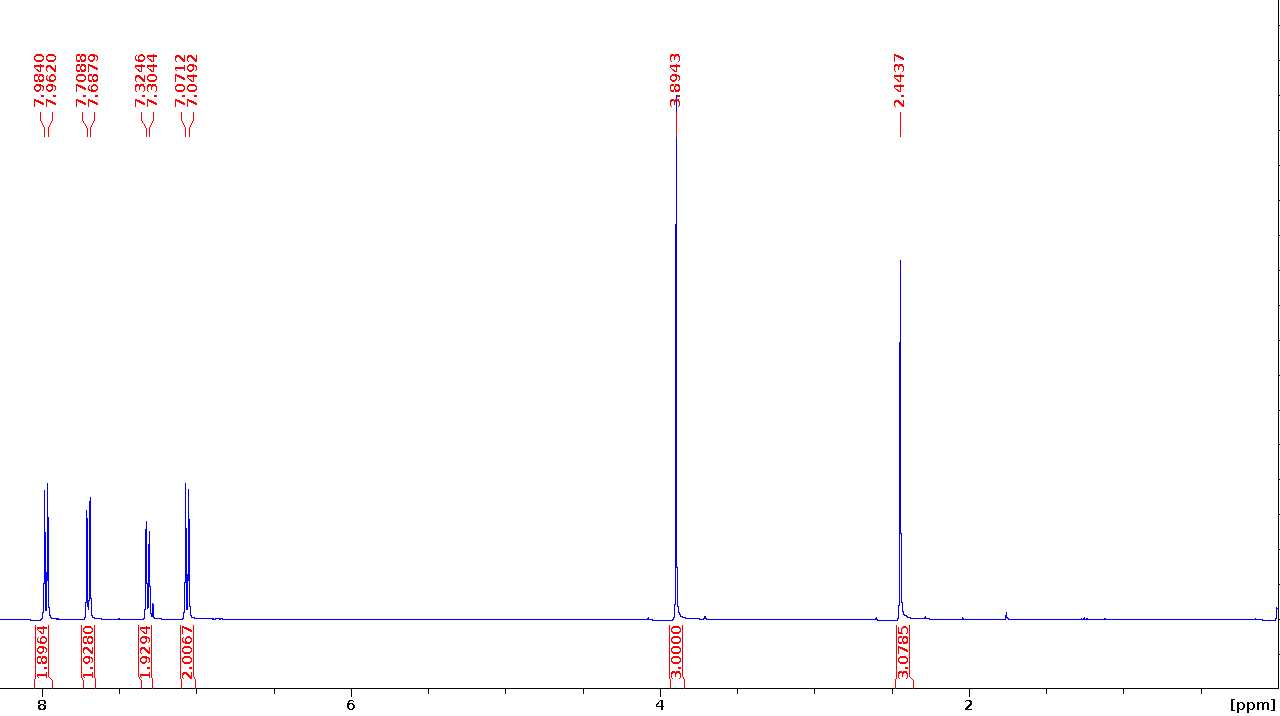
1 1H NMR (400 MHz, CDCl3) δ 7.98 (d, J=8.8 Hz, 2H), 7.71 (d, J=8.4 Hz, 2H), 7.32 (d, J=8.1 Hz, 2H), 7.07 (d, J=8.8 Hz, 2H), 3.89 (s, 3H), 2.44 (s, 3H).
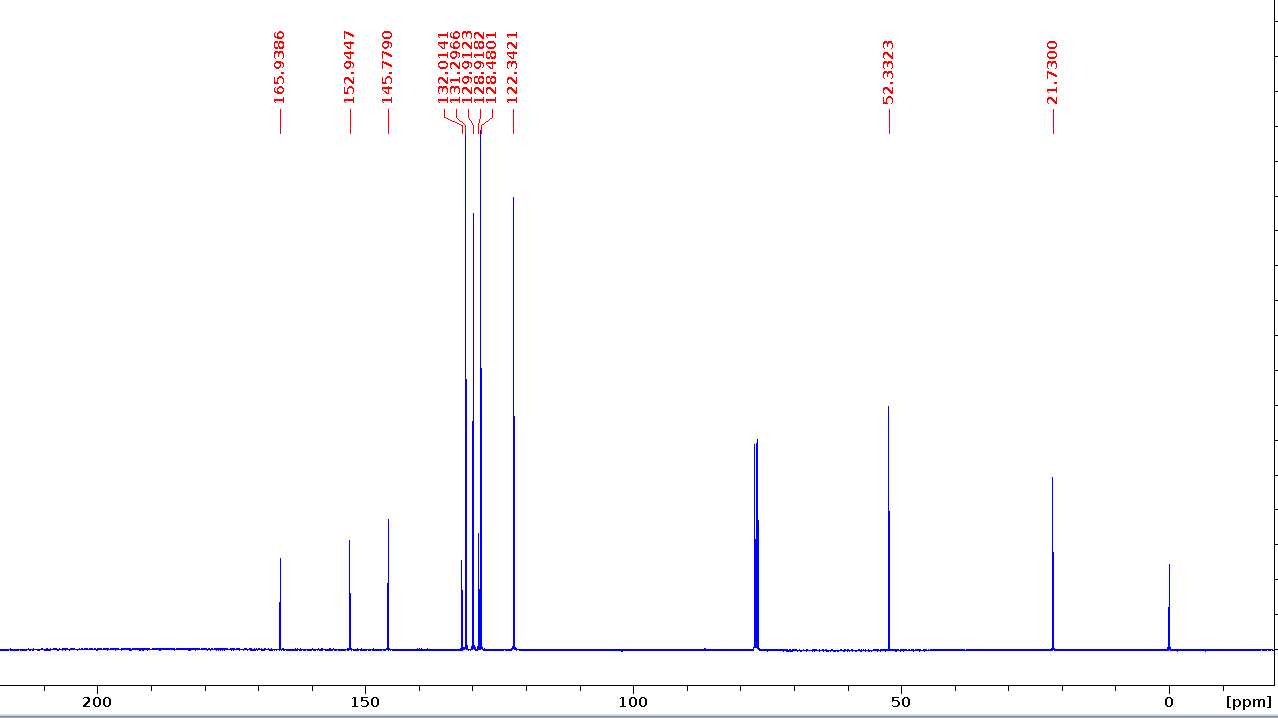
13C NMR (100 MHz, CDCl3) δ 165.9, 152.9, 145.8, 132.0, 131.3, 129.9, 128.9, 128.4, 122.3, 52.3, 21.7.
Methyl 3-methoxy-4-(tosyloxy)benzoate (G-OTs)
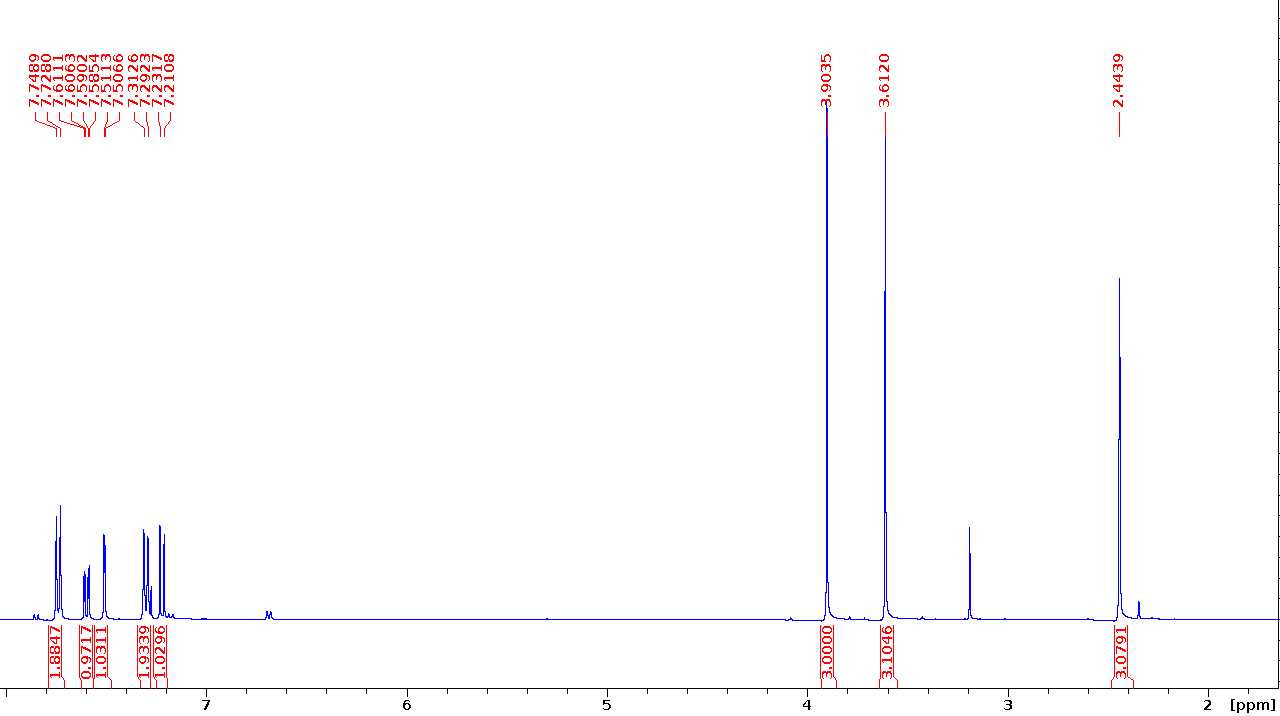
1 1H NMR (400 MHz, CDCl3) δ 7.75 (d, J=8.4 Hz, 2H), 7.61 (dd, J=6.4, 1.9 Hz, 1H), 7.51 (d, J=1.9 Hz, 1H), 7.31 (d, J=8.1 Hz, 2H), 7.23 (d, J=8.4 Hz, 2H), 3.90 (s, 3H), 3.61 (s, 3H), 2.44 (s, 3H).
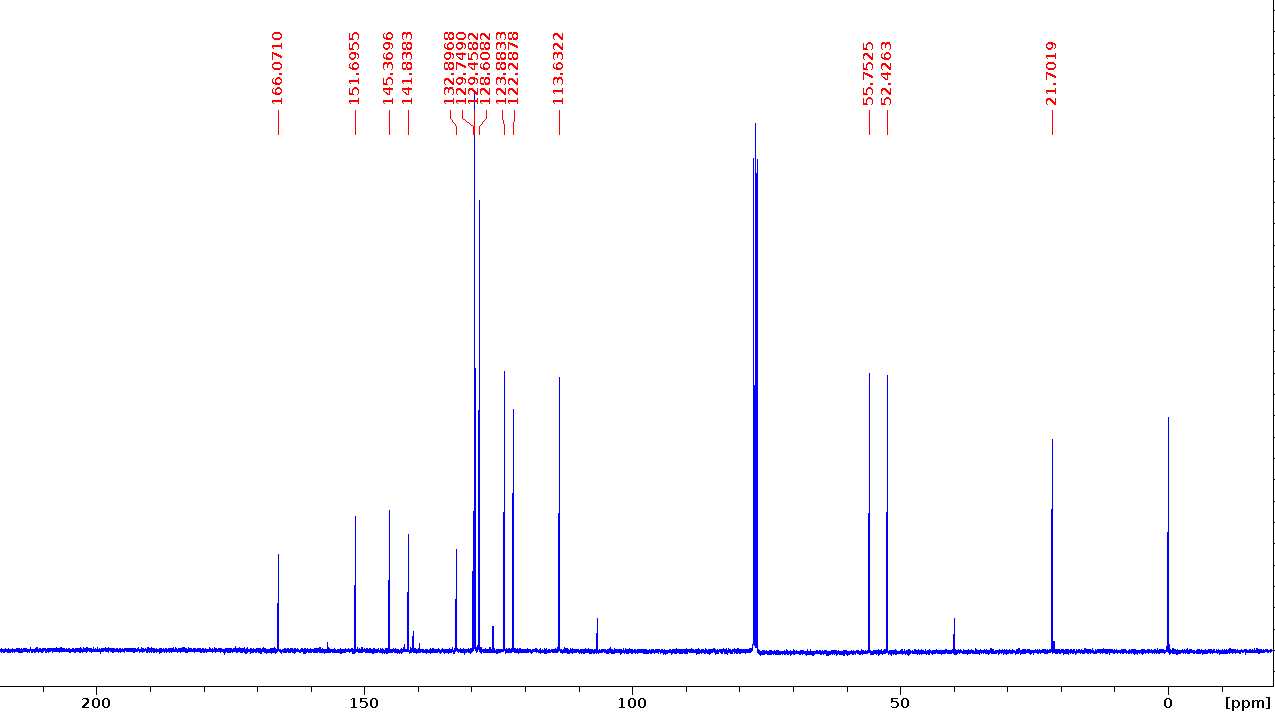
13C NMR (100 MHz, CDCl3) δ 166.1, 151.7, 145.4, 141.8, 132.9, 129.7, 129.5, 128.6, 123.9, 122.3, 113.6, 55.8, 52.4, 21.7.
Dimethyl 2-methoxy-4,4'-biphenyldicarboxylate (H-G dimer)
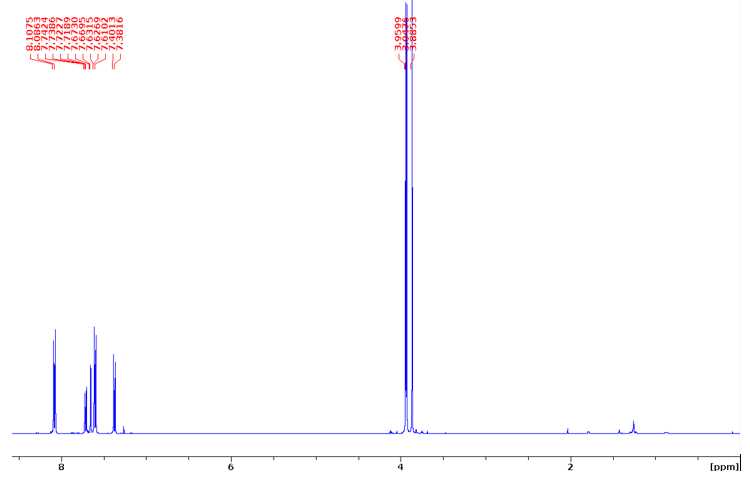
1 1H NMR (400 MHz, CDCl3) δ 8.11-8.09 (m, 2H), 7.74 (dd, J= 6.3, 1.5 Hz, 1H), 7.67 (d, J= 1.4 Hz, 1H),
7.63–7.61 (m, 2H), 7.40 (d, J= 7.9 Hz, 1H), 3.96 (s, 3H), 3.94 (s, 3H), 3.88 (s, 3H).
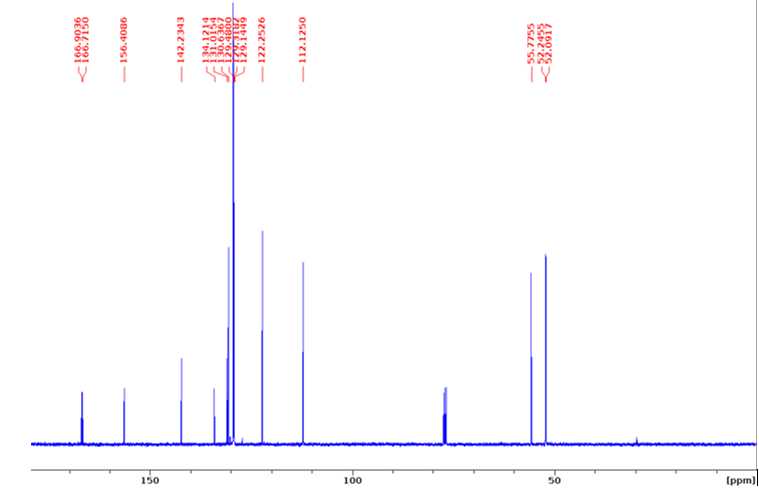
13C NMR (100 MHz, CDCl3) δ 166.9, 166.7, 156.4, 142.2, 134.1, 131.0, 130.6, 129.5, 129.3, 129.1, 122.3,
112.1, 55.8, 52.3, 52.1.
Dimethyl 2,2'-dimethoxy-4,4'-biphenyldicarboxylate (G-G dimer)
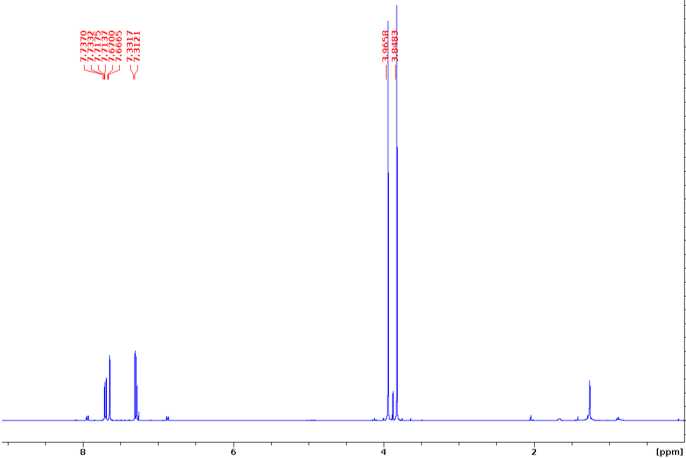
1HNMR (400 MHz, CDCl3)δ 7.74 (dd, J= 6.3, 1.5 Hz, 2H), 7.67 (d, J= 1.4 Hz, 2H), 7.33 (d, J= 7.8 Hz,
2H), 3.87 (s, 6H), 3.85 (s, 6H).
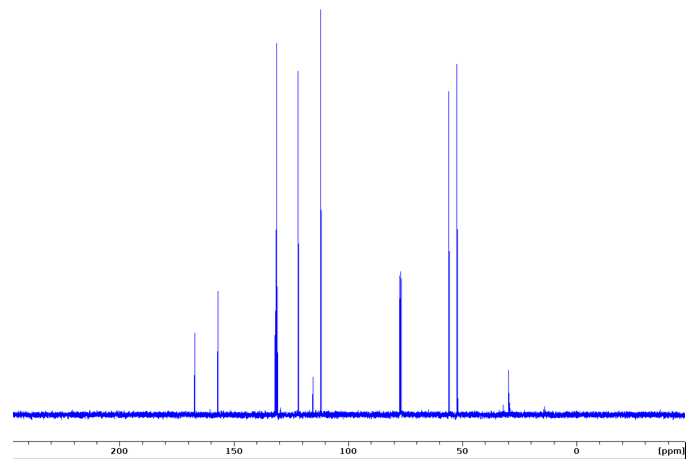
13CNMR (100 MHz, CDCl3)δ 166.9, 156.9, 131.8, 131.1, 130.9, 121.8, 111.9, 55.4, 52.2.
Biphenyl dimethyl dicarboxylate (H-H dimer)
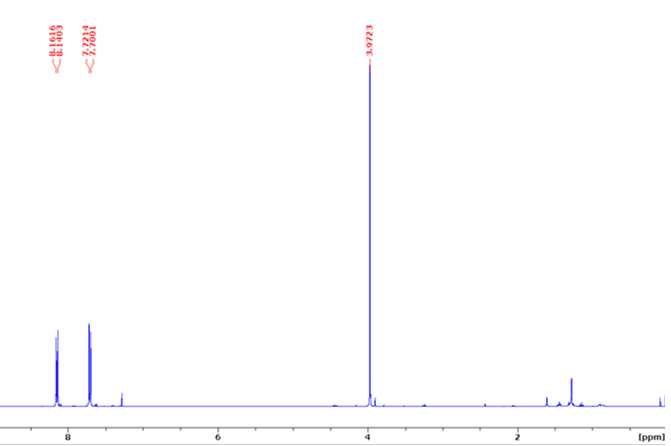
1HNMR (400 MHz, CDCl3)δ 8.16 (d, J= 8.5 Hz, 4H), 7.72 (d, J= 8.5 Hz, 4H), 3.97 (s, 6H).
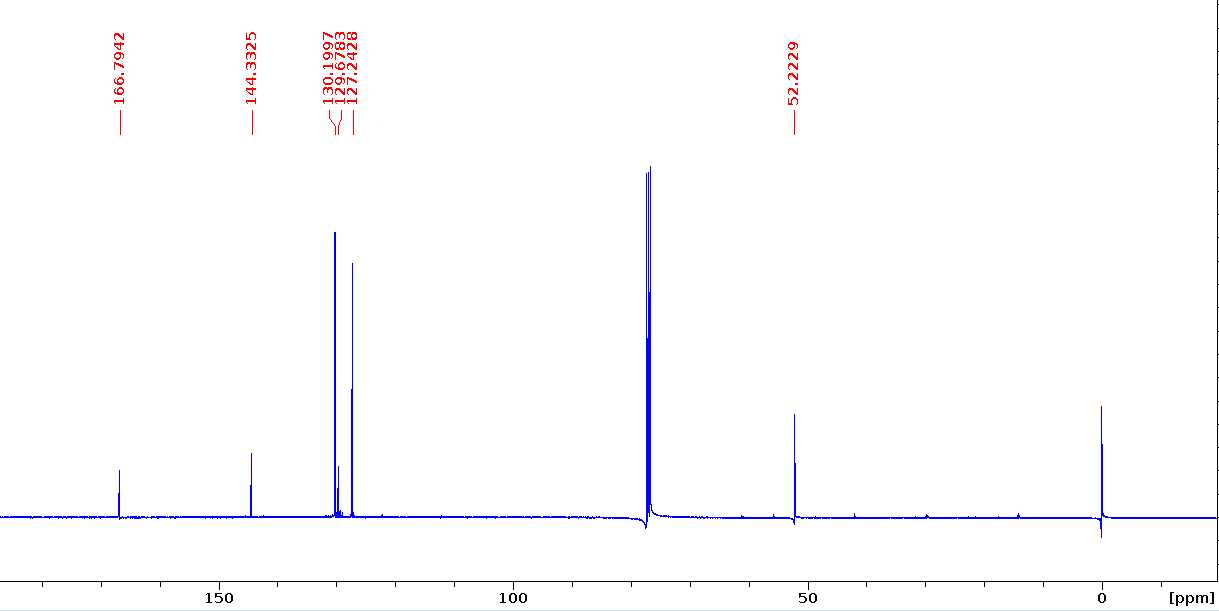
13C NMR (100 MHz, CDCl3) δ 166.8, 144.3, 130.2, 129.7, 127.2, 55.2.