Sonication of α-synuclein Fibrils for injection into the mouse brain
Vijay Singh, Marta Castellana-Cruz, Nunilo Cremades, Laura Volpicelli-Daley
Disclaimer
The protocols.io team notes that research involving animals and humans must be conducted according to internationally-accepted standards and should always have prior approval from an Institutional Ethics Committee or Board.
Abstract
Animal models that accurately recapitulate the accumulation of alpha-synuclein (α-syn) inclusions, progressive neurodegeneration of the nigrostriatal system and motor deficits can be useful tools for Parkinson's disease (PD) research. The preformed fibril (PFF) synucleinopathy model in rodents generally displays these PD-relevant features, however, the magnitude and predictability of these events is far from established. We therefore have optimized the sonication protocol of α-syn fibrils to ensure reliable, robust results. The protocol includes steps for re-sonication of PFFs on the day of injection into mice. The sonicated PFFs we received need to be re-sonicated to ensure that any fibrils aggregated due to long-term storage can be optimally fragmented to seed synuclein pathology once injected into mice.
Before start
Sonicating Fibril
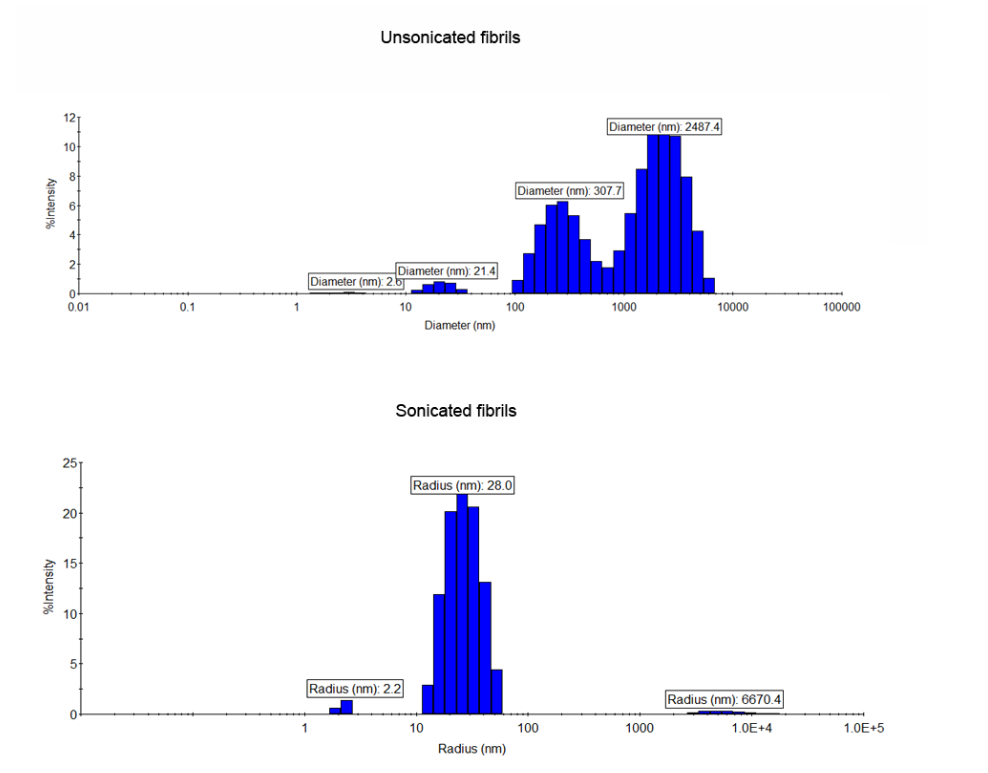
Proper sonication is a key step for the fibril model to work. For all in vivo work which involved injecting fibrils into mice or rats, we use the QSonica 700 sonicator with cup horn and tube rack for 1.5 mL polypropylene tubes with a chiller at 16°C. The cup horn sonication produces short fragments which maintain their morphology for 6-8 hours (at least) and can be stored in dry ice overnight, thawed and maintained at room temperature, and therefore remain active after overnight shipments.We found that over time, the heat generated by a probe tip sonicator causes the fibrils to form amorphous aggregates (Figure 1). This is a problem because stereotaxic surgeries can take several hours and the amorphous aggregates that form while the fibrils sit on the bench causes variability and reduces the concentration of seeding competent fragments. Another advantage of using the cup horn sonicator over probe tip is that 25 μL of fibrils can be sonicated, reducing the volume needed. This is also a closed tube system which increases safety. For neuron or cell culture work in which the fibrils are added to media and then the cells immediately after sonication, a probe tip sonicator is okay. Again, this should be performed in a BSL2 hood with all proper PPE (nanoparticle respirator, goggles, gloves etc.). The volume of fibrils to be sonicated cannot be less than 100 μL.
In all cases, we wear PPE when working with fibrils. We clean any spills with 1% SDS.

Attachments
Steps
Sonicating Fibrils
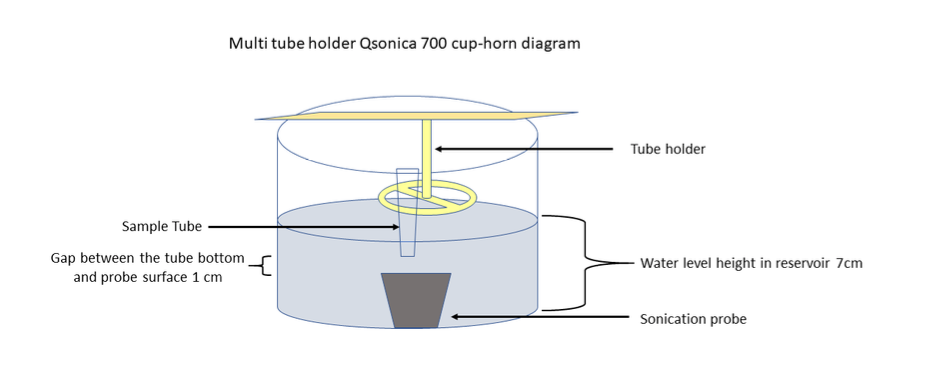
WePFFs Fill Qsonica water reservoir with about 900mL.
Attach cooling system to Qsonica and set the temperature at 10°C.
Thaw and pipet 25-22µL in 1.5mL polystyrene sonication tube.
Place 1.5mL sonication tube with fibrils in Qsonica tube holder.
Total sonication time: 0h 15m 0s.
Sonication pulse on for 3 s and off for 3 s
Amplitude 45%.
Sonicate sample for another 7:30 min only if we receive unsonicated PFFs.
Confirming Fragmentation
Dynamic Light Scattering:
Pipet 1µL and dilute with 4µL.
When done, fill cuvette with 2% SDS to decontaminate fibrils (using a squirt bottle).
Let sit for at least 0h 30m 0s.
Use squirt bottle to rinse several times with DI water.
Use filtered water for last rinse.
Make sure dry before next use.