Qualitative & Quantitative Assessment of Human Islets Using Dithizone (DTZ)
Human Islet Phenotyping Program (HIPP) of the IIDP
Abstract
This Standard Operating Procedure is adapted from the work of the 'National Institutes of Health-Sponsored Clinical Islet Transplantation Consortium Phase 3 Trial: Manufacture of a Complex Cellular Product at Eight Processing Facilities'' following the SOP cited in the document 'Purified Human Pancreatic Islet: Qualitative and Quantitative Assessment of Islets Using Dithizone (DTZ) – This Standard Operating Procedure is adapted from the work of the 'National Institutes of Health-Sponsored Clinical Islet Transplantation Consortium Phase 3 Trial: Manufacture of a Complex Cellular Product at Eight Processing Facilities'' following the SOP cited in the document 'Purified Human Pancreatic Islet: Qualitative and Quantitative Assessment of Islets Using Dithizone (DTZ) – Standard Operating Procedure of the NIH Clinical Islet Transplantation Consortium'
This SOP defines the assay method used by the Human Islet Phenotyping Program (HIPP) for quantitative and qualitative determination of the Purified Human Pancreatic Islet product, post-shipment, manufactured for use in the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)-sponsored research in the Integrated Islet Distribution Program (IIDP).
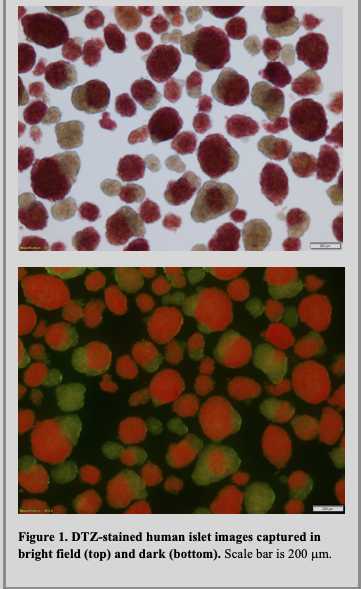
Dithizone (diphenyl thiocarbazone; DTZ) is an organic chemical that chelates the zinc in the insulin granules present in the beta cells of the pancreatic islets. The islet cells are stained red while the acinar cells remain unstained.
DTZ staining is used as a lot release and as an in-process assay:
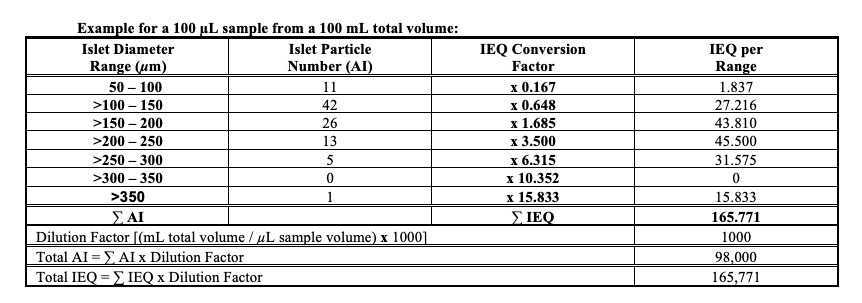
(i) Lot release testing: DTZ staining is used to identify islets and to determine the quantity and quality of the final islet product. Islet quantity is expressed as the number of islet equivalents (IEQ), which is calculated based on the number and diameter of the islets present in the preparation, mathematically corrected for islet volume.
(ii) In-process testing: DTZ staining is used to identify islets and to assess the effectiveness of the digestion, isolation and purification processes. The quality of the preparations is expressed as percent islet purity, and percent trapped islets. Islet quantity (IEQ) is also assessed.
Steps
Preparation of Working Dithizone
Assemble all items described in Materials section.
Prepare DTZ stain as described below. Observe all safety precautions when working with DMSO.
Wearing gloves, dissolve 50mg dithizone in 10mL DMSO.
Top up to 50mL with DPBS.
Filter the combined solution using a 0.45µm nylon filter.
Place solution in a 50mLconical tube and label “Dithizone Stain” with:
♦ Preparation Date and Time
♦ Expiration Date and Time (24 hours after preparation)
♦ Initials of person preparing solution
Triplicate samples for islet quantitation, grading, and purity should be obtained upon receipt of the islet shipment. Triplicate samples must be taken for each count and purity assessment. Islet grading should be performed by three certified personnel, but may be performed by two personnel if three are not available. Results will be averaged for final reporting on the IIDP website. If triplicate counts of IEQ are more than 30% different, then a fourth sample should be taken and the four averaged. Date should be documented on the worksheets and Excel files for each procedure.
Mix the final islet suspension very gently but thoroughly by inverting the islet prep in a conical 2-3 times before quickly taking a sample. (Do not swirl.) As islets settle rapidly, care must be taken to ensure a representative sample is taken. It is best to have two staff perform this procedure together, one mixing and the second taking the sample.
Take replicate 320µLsample volumes from 32mLtotal volume of shipment plus 680µLCMRL medium in 35 mm dishes.
Add 50µLof the DTZ solution to the islet sample and allow staining for 1 – 2 minutes at room temperature. Add an additional 1mLCMRL medium to plate before imaging.
Place a dish containing islets on stand of stereomicroscope equipped with a high-resolution camera, and swirl until all tissue is in the camera field of view at 10x magnification. Capture brightfield images at approximately 12-ms exposure and darkfield images at approximately 1.2-s exposure, each at 10x magnification. Ensure all tissue is present in image. Save all image files. Examples of islet images are shown in Figure 1.
Open the darkfield image in the cellSens software. Using the manual HSV threshold function, segment the islet tissue channel (stained red) and non-islet tissue (unstained).
Use the custom Count and Measure algorithm to determine islet count, mean islet diameter, and area of islet and non-islet tissue in the image. Split adjacent but discrete islets using the Manually Split Objects tool to get an accurate islet count (AI). For IEQ calculation, use the Object Filter to exclude islets <50µm in diameter. For Islet Purity calculation, turn off the Object Filter to include all tissue.
Calculate the dilution factor as follows:
Dilution Factor = Total volume of islet preparation that sample aliquot was taken from (mL) x 1000 / Volume of sample aliquot (µL)
The Islet Index (II), a calculation made by dividing the IEQ by the AI, will be calculated by the system when these parameters are entered into the system. This designation determines the overall size distribution of the islets being shipped.
Add up the Islet Ranking Points for each sample to obtain the Islet Quality Grade and record grade on Islet Cell Calculation Excel Worksheet. (See Attachment 1)
HIPP Protocol-07-IEQ_Counts_and_Islet_Ranking-Attachment_1_v3-3.xlsx
Deviations and Resolutions:
Document any deviations that occurred during this protocol that affect the final results and report with the analysis of the assay.
Data Storage and Reporting
Data Storage and Reporting
To facilitate data management and ensure data security, the Vanderbilt HIPP uses an institutional server-based platform for data storage and analysis.
Upon completion of image acquisition and analysis, annotated images containing metadata, image analysis outputs, and documentation of any deviations and repetitions that are warranted will be uploaded to the IIDP-HIPP database and disseminated to IIDP-affiliated investigators and islet isolation centers.