Hornwort (Anthoceros agrestis) biolistics-mediated transformation protocol
Declan Lafferty, Fay-Wei Li
Anthoceros agrestis
biolistics
bryophyte
fluorescent protein tagging
gene gun
hornwort
plant transformation
pyrenoid
Abstract
Here we report an efficient biolistics method for generating transient-expression and stable transgenic lines in the model hornwort, Anthoceros agrestis . An average of 569 (± 268) cells showed transient expression per bombardment, with green fluorescent protein expression observed within 48 hours. A total of 81 stably transformed lines were recovered across three separate experiments, averaging six lines per bombardment. We followed the same method to transiently transform nine additional hornwort species, and obtained stable transformants from A. fusiformis .
Steps
Growth media
For growing and maintaining gametophyte tissue, use AG medium (modified Hatcher’s medium containing additional 0.2% sucrose, 0.4 g ammonium nitrate, 100 mg/l activated charcoal, 5 mM MES buffering at pH 6.5 and 10 nM BA).AG medium for Hornwort solid and liquid culture.docx
For preparing tissue for transformation, use AG medium with high sucrose (2% sucrose).
For selection of transformed tissue, use AG medium with 0.2% sucrose and 10 mg/L hygromycin (for hygromycin selection) or other selection agent(s).
Tissue maintenance
Hornwort thallus tissue is maintained on 0.2% sucrose AG medium. 3-6 g of thallus tissue is homogenized and sub-cultured every 4-6 weeks
For homogenization, tissue is placed in a sterile 50 mL falcon tube with approx. 30 mL sterile water, supplemented with 30 µl of 300 mg/mL timentin.
Tissue is homogenized with a T18 digital ULTRA TURRAX homogenizer (IKA, Germany) for 5 - 10 s at 4000 rpm
Filter the tissue through a 50 µM cell strainer to remove excess mucilage and water. Rinse the tissue with 50 mL sterile filtered water and remove excess mucilage and water using the cell strainer.
Using a Nunc Cell scraper (179693, ThermoFisher Scientific, USA), plate on AG medium with 0.2% sucrose. Plate the tissue thinly and cover the majority (~80%) of the plate, as shown in Fig. 1.

Hornwort thallus tissue is grown at 22°C under a 16/8 h light/dark cycle with 6-25 µmol/m²/s light intensity provided by Ecolux XL Starcoat F32T8 XL SP30 ECO paired with F32T8 XL SP41 ECO fluorescence bulbs (General Electric, USA).
Tissue preparation for transformation
Hornwort thallus tissue is prepared 7 days prior to transformation. 2 g of healthy thallus tissue is homogenized, following the same method as described above. Healthy tissues are green with expanded thalli and minimal rhizoid growth, as in Fig 1. Poor quality tissues are yellowish green with many rhizoids observed growing upwards.
Homogenize tissue with a T18 digital ULTRA TURRAX homogenizer (IKA, Germany) for 5 - 10 s at 4000 rpm
When homogenizing tissue for transformation, be careful not to homogenize too much. This will damage the tissue and unhealthy tissue leads to poor transformation. Homogenize just enough to break up the tissue into small pieces, no clumps, approximately 5 seconds.
Filter the tissue through a 50 µM cell strainer to remove excess mucilage and water. Rinse the tissue with 50 mL sterile filtered water and remove excess mucilage and water using the cell strainer.
Plate tissue on 2% sucrose AG medium. If there is excess water or condensation on the plate, leave it open in a laminar flow hood to dry (approx. 20 min), as excess tissue moisture can negatively affect results. Draw a 2.5cm circle in the middle of the underside of the plate (I use the inside of a roll of micropore tape).
Plate approximately 0.15 g of wet-weight, homogenized tissue within the 2.5 cm bullseye mark (Fig. 2).
Using a cell scraper (179693, ThermoFisher Scientific, USA), plate the tissue as thinly as possible and leave open for another 10 mins to dry the tissue, to remove excess water. High moisture will decrease theefficiency of transformation. Don’t want to dry out the tissue too much though, as this will damage the tissue.
Tissue is left to recover for seven days. Tissue is grown using the same growth conditions as described in step 9.
Gold particle preparation (10 preps)
Weigh out 50 mg of gold particles (0.6 µm size, ideally from Biorad) in a 1.5 ml tube.
Add 1 ml of 70% ethanol and vortex vigorously for 3-5 minutes.
Allow the particles to soak in the liquid for 15 minutes.
Pellet the particles for 30 sec at 6000 rpm. Increase vortex time if the liquid is still cloudy.
Carefully discard the supernatant.
Repeat the following wash steps three times: Add 1 ml of sterile water, vortex for 1 min, allow particles to settle for 1 min, pellet the particles by briefly spinning them down and discard the supernatant.
Add 1 ml of sterile 50% glycerol, vortex vigorously for 1 min and aliquot into 100 µl portions in 1.5 ml tubes. Store at -20°C.
DNA/gold preparation (10 replicates)
The macrocarriers (#1652335, Bio-Rad, USA), 450 psi rupture discs (#1652326, Bio-Rad, USA), stopping screens (#1652336, Bio-Rad, USA) and macrocarreir holders are sterilized by soaking in 70% ethanol for at least 20 mins in a laminar flow hood, followed by drying until no ethanol remains.
Insert the sterilised macrocarrier in the the macrocarrier holder using sterilised forceps.
Vortex 100 µl of gold particles (0.6 – 1.0 µm size, 50 mg/ml concentration in 1.5 ml tube) at maximum vortex speed for at least 30 sec
Add 20 µl of 1 µg/µl DNA and vortex for 20 sec
Simultaneously add 100 µl of 2.5 M CaCl2, and 40 µl of 0.1 M spermidine free base and vortex for 1 min
Use fresh spermidine and store at -20°C for a maximum of four months.
Spin down the mixture at 6000 rpm for 8 sec. Carefully discard the supernatant by pipetting.
Add 180 µl of 70% ethanol to the gold particles in the tube. Dislodge the gold particles from the tube using a pipet tip and vortex at max speed for 20 sec.
Remove the supernatant and add 200 µl of 100% ethanol. Dislodge the gold particles from the tube using a pipet tip and vortex at max speed for 20 sec.
Spin down the gold particles at 6000 rpm for 8 sec. Remove about 145 µl of supernatant. Resuspend the gold particles in the remaining ethanol by vortexing at max speed for 20 sec.
Quickly spread about 5 µl of the gold particle suspension onto each flying disk that was previously sterilized and mounted on the flying disk carrier under laminar flow hood, mixing the gold suspension well in between the spreading, using the sonicator. 10 flying disks macrocarriers may be prepared this way. Let it dry for at least 30 min prior to bombardment.
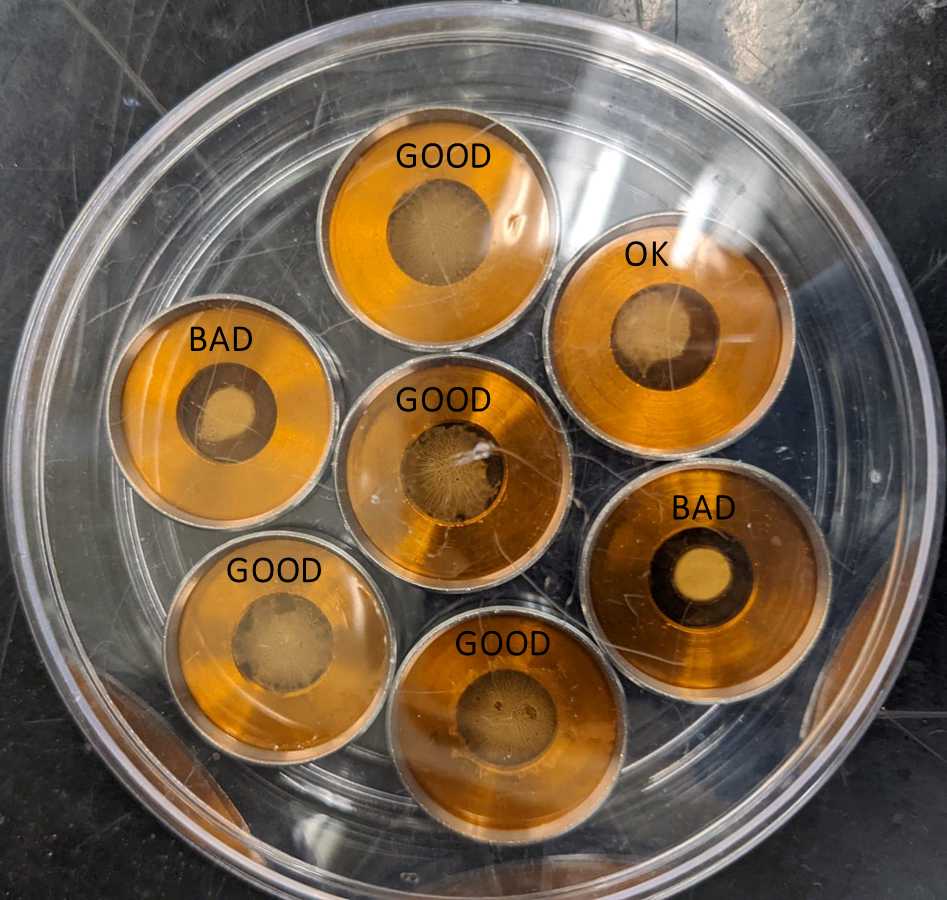
It is important that the gold/ethanol mix spreads evenly over the macrocarrier (Fig. 3). Clumping of gold particles will negatively impact the efficiency of transformation.
I use a sonicator to keep the gold/DNA mix homogenous. Can also use a vortex.
Biolistics-mediated transformation of hornwort thallus tissue
While the gold/DNA mix drys, sterilise the Biorad PDS-1000 (#1652257, Bio-Rad, USA) chamber and the laminar flow hood with 3% bleach, followed by 70% ethanol. Allow equipment to dry.
Start the Biorad PDS-1000 and the vacuum pump. Ensure helium pressure line is set at ~600 psi
We have our Biorad PDS-1000 within a laminar flow hood, to reduce potential contamination
Shoot a blank shot under 30 psi vacuum to clear the helium line.
Clean the bombardment chamber using 70% ethanol and sterilized paper towels.
Place the 2.5 cm diameter homogenized tissue inside the bombardment chamber at the lowest position. Remove the petri dish lid.
Let the chamber vacuum pressure reach approximately 29 inHg before engaging the gun.
After shooting, immediately place the petri dish lid back and then remove from the chamber.
If different DNA constructs are bombarded for different samples, clean the bombardment chamber using 70% ethanol and sterilized paper towels prior to shooting. Ensure the chamber is fully dried prior to resuming bombardments.
Once all samples have been shot, seal the edges of the closed petri dish lid using double layer of parafilm.
Selection of transformants
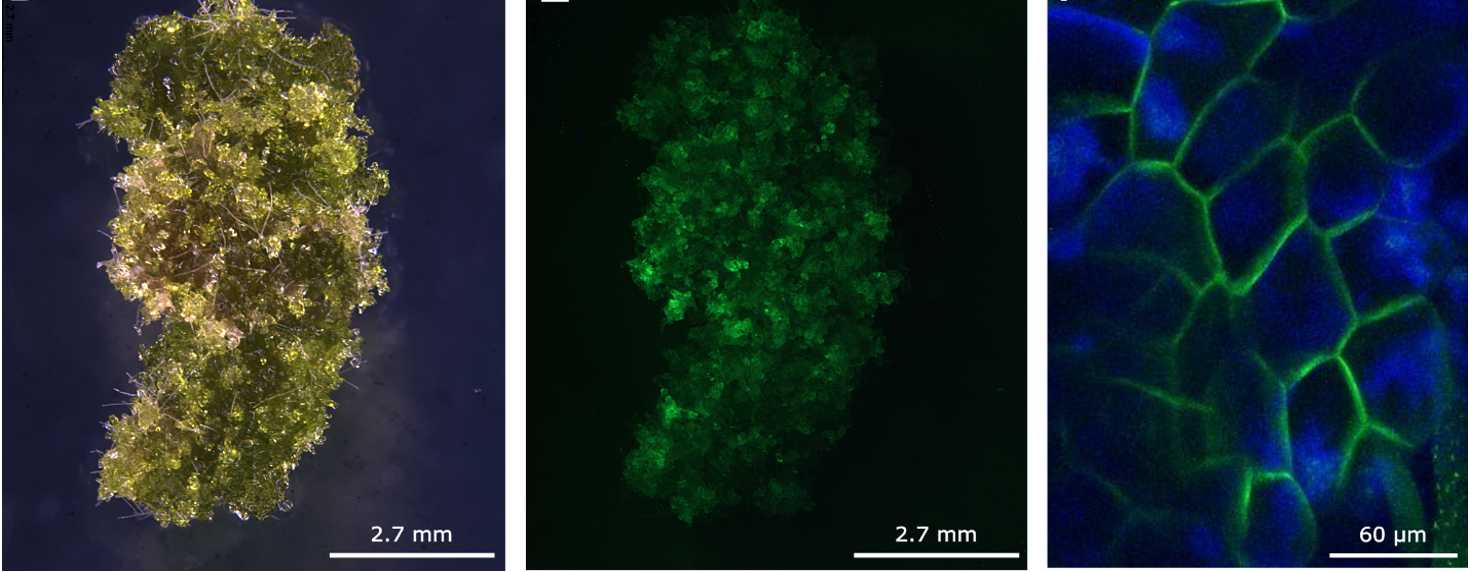
If using fluorescent markers regulated by strong promoters (eg. 35S or EF1a promoters), transient gene expression can be observed after 24 hr post bombardment, and can continue until more than 3 weeks post bombardment (Fig. 5). Most transient expression will disappear within 2 weeks post bombardment.

For stable transformation, tissue is recovered for 7 days post-bombardment. Transformed tissue is then pooled and homogenized, with a maximum of 5 replicates per homogenization (or else there is too much tissue for the homogenizer).
To homogenize the tissue, follow the same method as described in steps 11 and 12. It is very important not to homogenize for too long, only until the main tissue clumps are broken up, about 3-5 seconds.
Filter the tissue well, removing all mucilage and rinsing the tissue until the flow through is clear
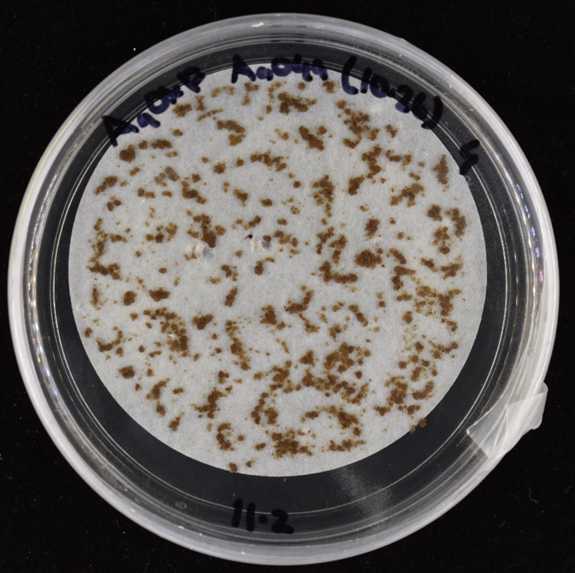
Using a cell scraper, place a small amount (0.15-0.3 g) of tissue onto 0.2% AG solid media, without selection, overlaid with sterilised 70 mm diameter Whatman filter paper (WHA1001070, Sigma-Aldrich, USA) (Fig. 6). Grow in the same conditions described in step 9.

I tend to double the plates i.e if I homogenized transformed tissue from five plates, then I will prep ten plates for the filter paper step
Can add ~1 mL of sterile MQ water to the tissue to help it spread out nicely, the aim is to avoid clumps of tissue.
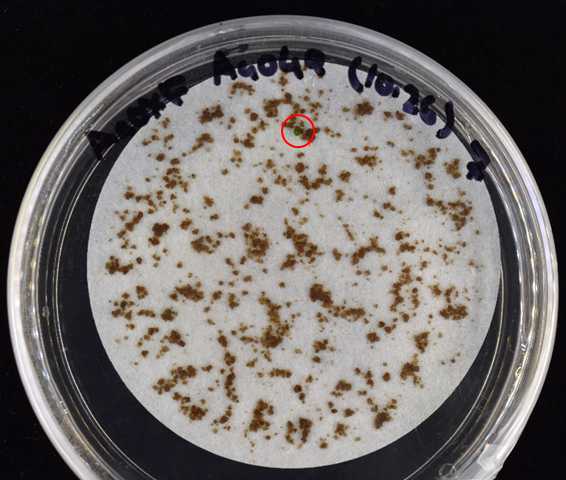
Three days post homogenization, transfer the filter paper to 0.2% AG + 10 mg/L hygromycin solid media. Grow in the same conditions described in step 9.
When using other selectable markers it may be necessary to transfer to fresh medium after 5 weeks on selection. This is not required for hygromycin selection.
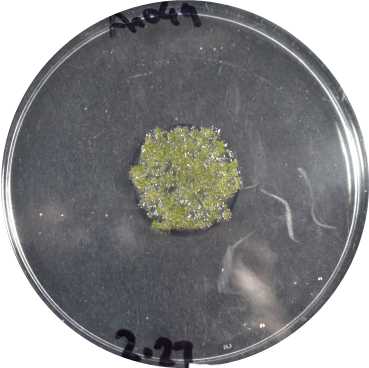
Transfer the regenerating tissue to new plates to separate it from non-transformed, dying tissue.
If using a fluorescent marker, the presence of the transgene can be confirmed using fluorescent microscopy (Fig. 9). Otherwise, continue to sub-culture the stably transformed lines until there is sufficient tissue for DNA extraction. Presence of the transgene can be confirmed via PCR or DNA sequencing.