Generation of Immunodeficient Mice Bearing Human Immune Systems by the Engraftment of Hematopoietic Stem Cells
Suheyla Hasgur, Ken Edwin Aryee, Leonard D. Shultz, Dale L. Greiner, and Michael A. Brehm
Disclaimer
Abstract
Immunodeficient mice are being used as recipients of human hematopoietic stem cells (HSC) for in vivo analyses of human immune system development and function. The development of several stocks of immunodeficient Prkdc scid ( scid ) scid ( scid ), or recombination activating 1 or 2 gene ( Rag1 or Rag2 ) knockout mice bearing a targeted mutation in the gene encoding the IL2 receptor gamma chain ( IL2r γ ) have greatly facilitated the engraftment of human HSC and enhanced the development of functional human immune systems. These “humanized” mice are being used to study human hematopoiesis, human-specific immune therapies, human-specific pathogens, and human immune system homeostasis and function. The establishment of these model systems is technically challenging, and levels of human immune system development reported in the literature are variable between laboratories. The use of standard protocols for optimal engraftment of HSC and for monitoring the development of the human immune systems would enable more direct comparisons between humanized mice generated in different laboratories. Here we describe a standard protocol for the engraftment of human HSC into 21-day old NOD-scid IL2r γ ( NSG) mice using an intravenous injection approach. The multi-parameter flow cytometry used to monitor human immune system development and the kinetics of development are described.
ACKNOWLEDGEMENT
This work was supported by National Institutes of Health Grants AI046629, AI112321, DK104218, CA034196, and OD018259 and grants from the Helmsley Charitable Trust and the Juvenile Diabetes Research Foundation. The authors would like to thank to TUBITAK (The Scientific and Technological Research Council of Turkey) for the research support (2214A). MAB and DLR are consultants for the Jackson Laboratory (Bar Harbor, ME).The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Before start
The protocols described below involve the manipulation of primary human cells. All work should be done in a standard laminar flow hood and with appropriate personal protective equipment. Waste materials should be disposed of using protocols approved by an Institutional Biosafety Committee. All mouse injections and handling should be done using protocols approved by an Institutional Animal Care and Use Committee.
Steps
Methods
Preparation of umbilical cord blood (UCB)
Allow histopaque and RPMI supplemented with 0.5% BSA to warm to room temperature.
Remove buffy coat layer, transfer to a new 50mL conical tube.
Add 30mL of RPMI supplemented with 0.5% BSA and centrifuge for 5 min 400 × g and 4°C .
Discard supernatant
Pool pellets from each 50mL conical tube in a total volume of 10 mls of RPMI supplemented with 0.5% BSA. ( See NOTE 4 )
Perform viability count.
Reserve 100,000 cells for flow cytometric analysis.
Centrifuge cells for 5 min at 400 × g and 4°C and proceed to step 2.
Transfer UBC to 50mL conical tubes (30 mls per tube).
Add hespan to each tube of UBC to a final concentration of 20% per volume and mix gently.
Incubate for 30 minutes at room temperature. The incubation period is to allow red blood cells (RBC) to sediment.
Remove bottom layer of RBC with 10mL pipette, leaving approximately 2mLof the RBC volume.
Add RPMI supplemented with 0.5% BSA to each tube to bring to a total volume of 30mL.
Slowly underlay 14mLof histopaque to each tube containing umbilical cord blood. Ensure a clear interface is maintained between the histopaque and the cell-containing medium.
Spin for 30 min at 300 × g at room temperature with centrifuge brake off.
Remove the top layer of plasma, leaving 2mLvolume above buffy coat layer.
Depletion of CD3+ T cells
Our laboratory uses reagents from Miltenyi Biotech to deplete human CD3+ T cells from UCB samples. The manufacture’s protocol was followed for depletion of human T cells ( See NOTE 2 )
Resuspend CD3-depleted UCB cells in 1 ml RPMI supplemented with 0.5% BSA and perform viability counts.
To validate depletion of CD3+ T cells, stain recovered cells with antibodies specific for human CD3 and human CD45. T cell levels (cells staining double positive for CD3 and human CD45) should be below 0.5% of total cells. Cells saved from step 1.15 should be used as a positive staining control.
To determine the CD34+ HSC levels, stain the recovered cells with antibodies specific for human CD34 and human CD45. HSC are identified as CD45 dim and CD34 positive.
Resuspend cells in RPMI supplemented with 0.5% BSA. Keep cells on ice until injection.
HSC injection
The protocol for injecting newborn mice with HSC has been described in detail previously (6). Here we will focus on intravenous injection of HSC into 21 to 28 day old mice.
Irradiate recipient mice by whole body gamma irradiation. For young (21 to 28 days old) NSG mice a conditioning dose of 100 cGy is normally well tolerated and enables efficient engraftment. For engraftment of newborn NSG mice, 100 cGy is also used. HSC injection should be performed between four and 24 hours after irradiation.
Warm recipient mice using warming lamp.
Inject CD3-depleted UCB cells to obtain a total of 1×105CD34+ cells per injection into the lateral tail vein. For newborn mice show in here, 5×104CD34+ cells were injected by intracardiac route into 24 to 72 hour old NSG pups.
Ensure that bleeding from injection site has stopped and return recipient mouse to cage.
Evaluation of Human Cell Chimerism Levels by Flow Cytometry
HSC-engrafted NSG mice are normally screened for human cell chimerism levels in peripheral blood between 12 and 15 weeks post-injection. This evaluation is easily done by flow cytometric analysis of peripheral blood cells to validate human immune system development. For analysis, all cells positive for mouse CD45 are excluded from the gating strategy. Human immune cells are identified as staining positive for human CD45 and subpopulations are defined from those cells. The immune cell subsets that develop will be dependent on age of the recipient mouse and the specific time point evaluated. The results shown below compare human immune system development between HSC-engrafted newborn NSG mice and HSC-engrafted young (21-day old) NSG mice.
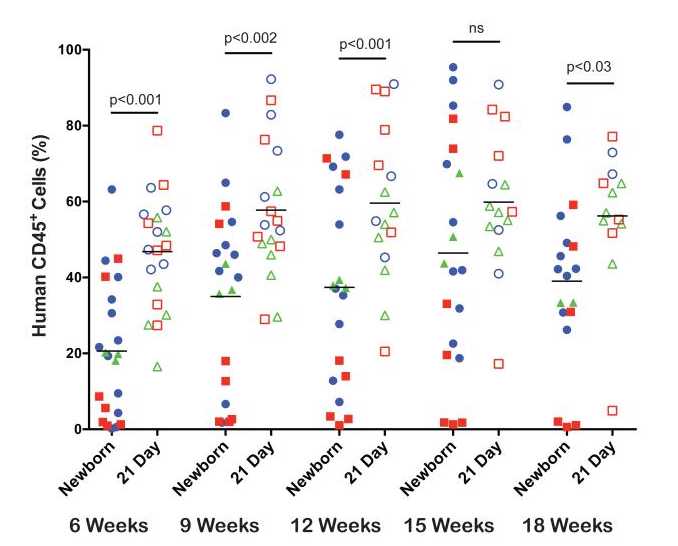
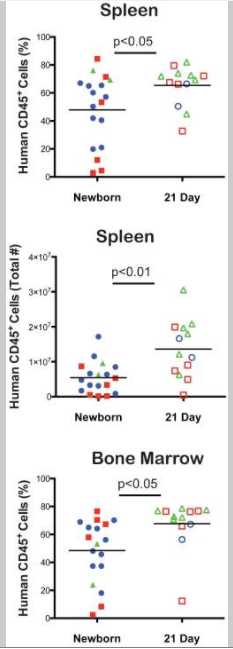
Levels of human CD45+ cells. Newborn and 21-day old NSG mice were irradiated and injected IV by intracardiac route (newborn) or via the tail vein (3 weeks old) with T cell depleted UCB containing 1×105CD34+cells as described above. Between 6 to 18 weeks after HSC injection, the percentages of human CD45+ cells in the blood were determined by flow cytometry (Figure 1). Each shape represents an individual animal and mice injected with the same HSC are identified by symbols of similar shape and color. Higher proportions of human CD45+ cells were detected in the blood of 21-day old, HSC-engrafted NSG mice at 6, 9,12 and 18 weeks as compared to newborn mice (Figure 1). In addition higher levels of human CD45+ cells were detected in the spleen and bone marrow of the 21-day old NSG mice compared with levels of circulating cells (Figure 2). These results show that 21-day old NSG mice and newborn NSG mice support HSC engraftment and that 21-day old mice show faster kinetics of human immune system development.
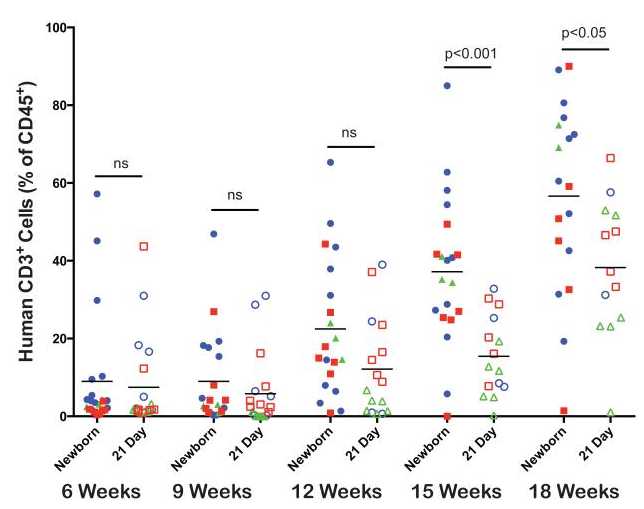
Human T cell development. The kinetics of human CD3+ T cell development were compared between newborn and 21-day old NSG mice that had been injected with human HSC as described above (Figure 3.) The levels of human T cells detected in the peripheral blood were low at the 6 and 9 week time points for both groups of HSC-engrafted NSG mice and began to increase by week 12. At weeks 15 and 18 the levels of T cells were significantly higher in newborn NSG mice as compared to 21-day old NSG mice. These data suggest that both newborn and 21-day old NSG mice support human T cells development, but the kinetics are accelerated in mice injected as newborns ( See NOTE 5 )
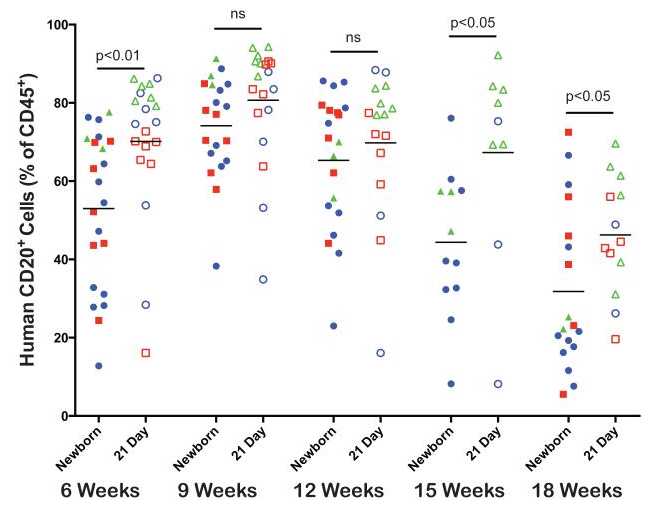
B cell development. The kinetics of human CD20+ B cell development were compared between newborn and 21-day old NSG mice that had been injected with human HSC as described above (Figure 4). Both newborn and 21-day old injected NSG mice supported B cell development and significantly higher levels were detected in 21-day old mice at 6, 15 and 18 weeks. Overall a significant proportion of the CD45+ cells in NSG mice were human B cells at all time points.
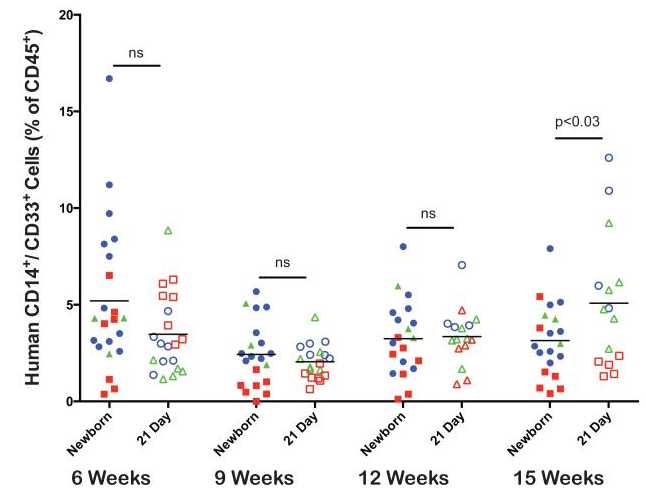
Monocyte/Macrophage development. The kinetics of human CD14+/CD33+ monocyte/macrophage development were compared between newborn and 21-day old NSG mice that had been injected with human HSC as described above (Figure 5). Both newborn and 21-day old NSG mice supported low levels of human monocyte/macrophage development with slightly higher levels detected in 21-day old mice at the 15 week time point. At all time points tested CD14+/CD33+ cells were detectable in the peripheral blood.