Assessment of Human Islet Composition and Acinar Cell Component by Immunofluorescence Staining
IIDP-HIPP
Abstract
This Standard Operating Procedure (SOP) is based on the Human Islet Phenotyping Program of the IIDP Immunofluorescence Staining Procedure. This SOP provides the HIPP procedure for immunofluorescent staining, imaging, and analysis of islet preparations.
This SOP defines the assay method used by the Human Islet Phenotyping Program (HIPP) for quantitative and qualitative determination of the Purified Human Pancreatic Islet product, post-shipment, manufactured for use in the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)-sponsored research in the Integrated Islet Distribution Program (IIDP).
Before start
Steps
Preparation of Reagents
Preparation of Reagents for Immunofluorescence Staining of Islet Cryosections
10% Triton X-100 stock ( ) 30mL) – combine 3mL Triton-X-100 and 27mL 1X PBS. Mix on shaker for 30 min or until Triton X-100 is completely dissolved and store at 4°Cfor up to 1 month.
Permeabilization Solution (0.2% Triton, 50mL ) – combine 1mLof 10% Triton stock and 49mL 1X PBS.
Blocking Buffer (5% NDS, ) 4mL) – combine 0.2mLNDS and 3.8mL1X PBS.
Antibody Buffer ( ) 10mL) – combine 0.1g BSA, 0.1mL 10% Triton stock, and 9.8mL 1X PBS.
DAPI staining solution (1:25,000, 50mL ) – combine 2µL DAPI stock (5mg/mL) and 50mL 1X PBS.
Procedure
Immunofluorescence Staining Procedure on Islet Cryosections
Use freshly-made antibody incubation buffers, and wash buffers. Steps 2.3, 2.4, 2.8, 2.10, 2.11 can be done in Kartell Staining Chambers.
Add secondary antibodies ( Table 1 ) diluted in 0.1% Triton/1% BSA/1X PBS and incubate for 1.5 hours at room temperature in a humidified chamber.
Aspirate the secondary antibody and counterstain slides with 1:25,000 DAPI/PBS for 10 minutes at room temperature.
Remove from DAPI and wash the sections with 1X PBS three times for 15 minutes each.
Mount the sections with SlowFade Gold mounting medium.
Let the frozen sections thaw at room temperature and air-dry for about 30 minutes.
Wash the sections with 50mL 1X PBS 3 times for 5 minutes to remove the OCT.
Permeablize the tissue section with 0.2 % Triton for 15 minutes at room temperature.
Wash the tissue in 50mL 1X PBS 3 times for 3-5 minutes.
Draw circles or rectangles around the sections with PAP marker and let them dry for about 5 minutes.
Block the sections with 5% normal donkey serum (made from 100% stock)/1X PBS at room temperature for 90 minutes in a humidified chamber.
Aspirate the blocking solution, add primary antibodies ( Table 1 ) diluted in 0.1% Triton-X-100 (made from 10% Triton stock)/1% BSA/1X PBS and incubate in a humidified chamber overnight at 4°C .
Table 1. List of primary and secondary antibodies for assessment of islet cell composition and endocrine/acinar cell composition 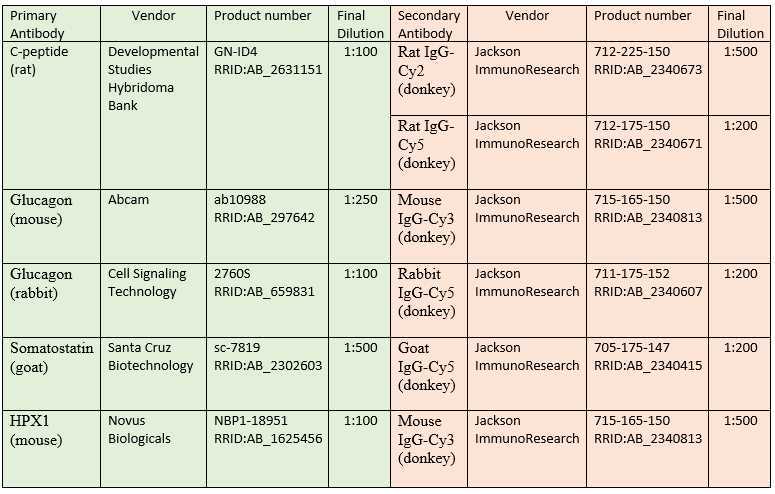
Aspirate the primary antibodies and wash the sections with 1X PBS three times for 10 minutes each.
Imaging and Analysis
Imaging and Analysis of Fluorescently Labeled Islet Cryosections
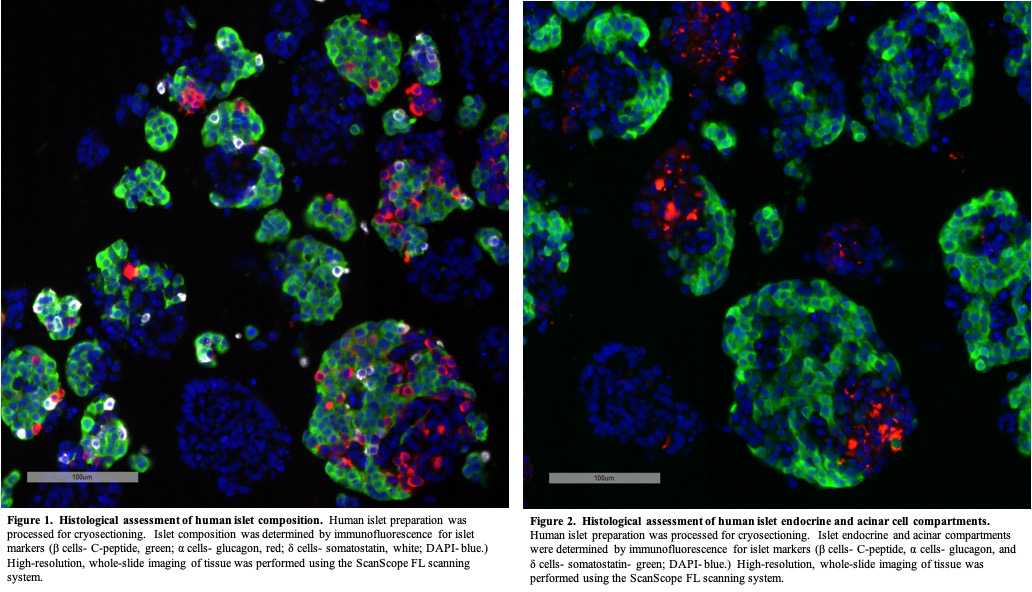
Capture images of islet sections using a high-resolution whole slide scanning system (ScanScope FL, Aperio/Leica) connected to a web-based digital slide repository powered by eSlide Manager and housed in the Vanderbilt University Medical Center data center ( examples of islet images are shown in Figures 1 Figures 1 and 2 ).
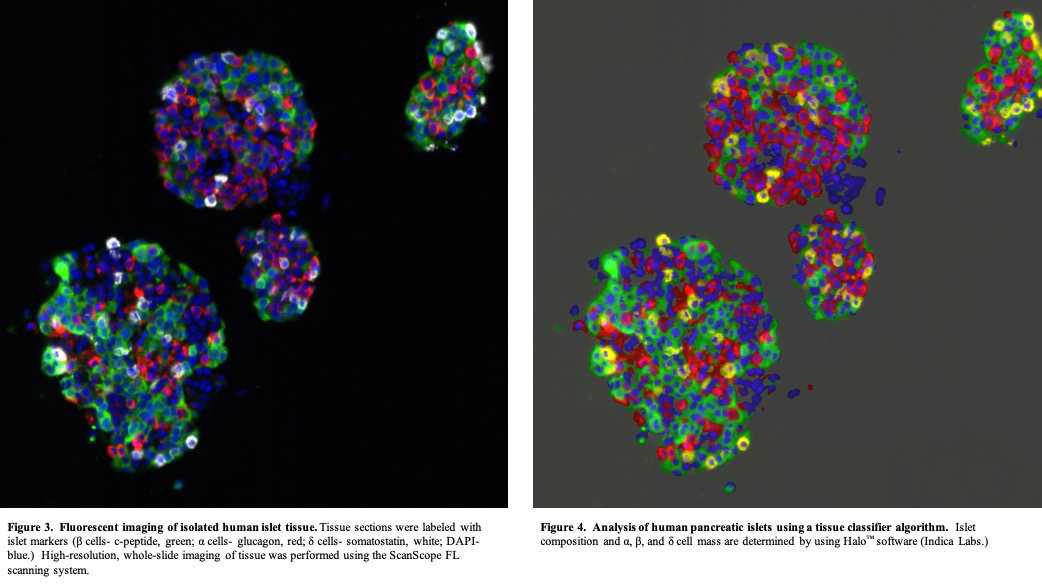
Using a tissue classifier algorithm (Halo™, Indica Labs) analyze islet images (50 -100 islets/labeling experiment) to provide a quantitative assessment of the islet cell composition and endocrine/acinar cell compartments for a given human islet preparation ( examples of quantitative islet assessment are shown Figures 3 and 4 Figures 3 and 4 ).
Data Storage and Reporting
Data Storage and Reporting
To facilitate data management and ensure data security, the Vanderbilt HIPP uses an institutional server-based platform for data storage and analysis.
Upon analysis completion (within 14 business days) annotated images containing metadata and image analysis outputs will be uploaded to the IIDP-HIPP database and immediately disseminated to IIDP-affiliated investigators and islet isolation centers.

