An ELISA-Based Method to Measure Mucosal Antibody Responses Against SARS-CoV-2 in Human Saliva
Gagandeep Singh, Gagandeep Singh, Disha Bhavsar, Disha Bhavsar, Kaori Sano, Kaori Sano, Florian Krammer, Florian Krammer
Abstract
The primary mode of transmission for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is infection of the respiratory tract through droplets and/or aerosols. Therefore, immune responses at respiratory mucosal surfaces play a significant role in the prevention of infection. Greater emphasis is now being placed on mucosal immunity induced by exposure to SARS-CoV-2 antigens through infection or vaccination. In concert with cellular immunity, humoral responses at mucosal surfaces, especially the secretory version of immunoglobulin A (sIgA), can be instrumental in preventing respiratory infections. A better understanding of mucosal immune responses can further our knowledge of immunity to SARS-CoV-2 and help inform vaccine design. Here we describe a detailed protocol for an in vitro assay based on the enzyme-linked immunosorbent assay (ELISA) to assess mucosal antibody response to SARS-CoV-2 spike protein in human saliva. © 2024 The Authors. Current Protocols published by Wiley Periodicals LLC.
Basic Protocol : ELISA measurement of mucosal antibodies to SARS-CoV-2 spike protein in human saliva
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in late 2019 and is the causative agent of coronavirus disease 2019 (COVID-19; Wu et al., 2020; Zhu et al., 2020). It has led to a global pandemic, causing significant morbidity and mortality across the globe. SARS-CoV-2 primarily infects the mucosal epithelium of the respiratory tract, potentially leading to pneumonia and other clinical manifestations of COVID-19 (Martines et al., 2020). As a result of this, the immune responses at the mucosal surfaces of the airways can serve as the first line of defense against infection. In particular, the secretory version of immunoglobulin A (sIgA) antibodies present in the respiratory airways can play a key role in offering protection. These antibodies also typically take the form of dimers, which have been shown to have higher antiviral activity than their monomeric counterparts (Suzuki et al., 2015). Mucosal antibody titers have also been associated with protection from infection with SARS-CoV-2 in humans (Havervall et al., 2022; Sheikh-Mohamed et al., 2022; Marking et al., 2023) and animal models (Sun et al., 2021). Mucosal immunity may be generated by exposure to the antigen at the mucosal surface, either through infection or through vaccine administered via the intranasal route. Current approved SARS-CoV-2 vaccines are mostly administered intramuscularly and thus do not elicit significant mucosal responses. However, in some individuals with prior history of infection, initial vaccination with mRNA vaccines has shown to elicit mucosal antibody responses (Sano et al., 2022).
Here, we present a protocol to measure mucosal antibody response to the spike (S) protein of SARS-CoV-2 by measuring spike-specific immunoglobulin G (IgG) and sIgA antibodies in saliva. We believe that the results from this assay can provide information about the level of mucosal immunity against SARS-CoV-2 in the general population and highlight the need to promote and boost mucosal immunity. We have taken into consideration previously reported results that the amount of IgA in saliva varies depending on several other factors, such as temperature, stress, and diurnal patterns (Brandtzaeg, 2007). Hence, the protocol includes analysis methods that adjust the amount of spike-specific sIgA secreted in saliva based on the total concentration of IgA in the saliva sample. This allows for a measure of mucosal immunity independent of the variations caused by other factors that typically confound the quantification of sIgA in saliva. In addition, monomeric IgA and IgG are known to leak into saliva through the gingival crevicular fluid. To ensure that only multimeric sIgA is detected, a detection antibody specific to the secretory-component-bound IgA is used. Thus, the current protocol comprises three experimental steps: an enzyme-linked immunosorbent assay (ELISA) to measure anti-S IgG, an ELISA to measure anti-S sIgA, and a total IgA (tIgA) quantification ELISA. An overview is shown in Figure 1. We believe that this method provides a practical way to measure mucosal immunity generated against SARS-CoV-2, and we have used it repeatedly to analyze immune responses to SARS-CoV-2 vaccination and infection (Bhavsar et al., 2023; Sano et al., 2022).
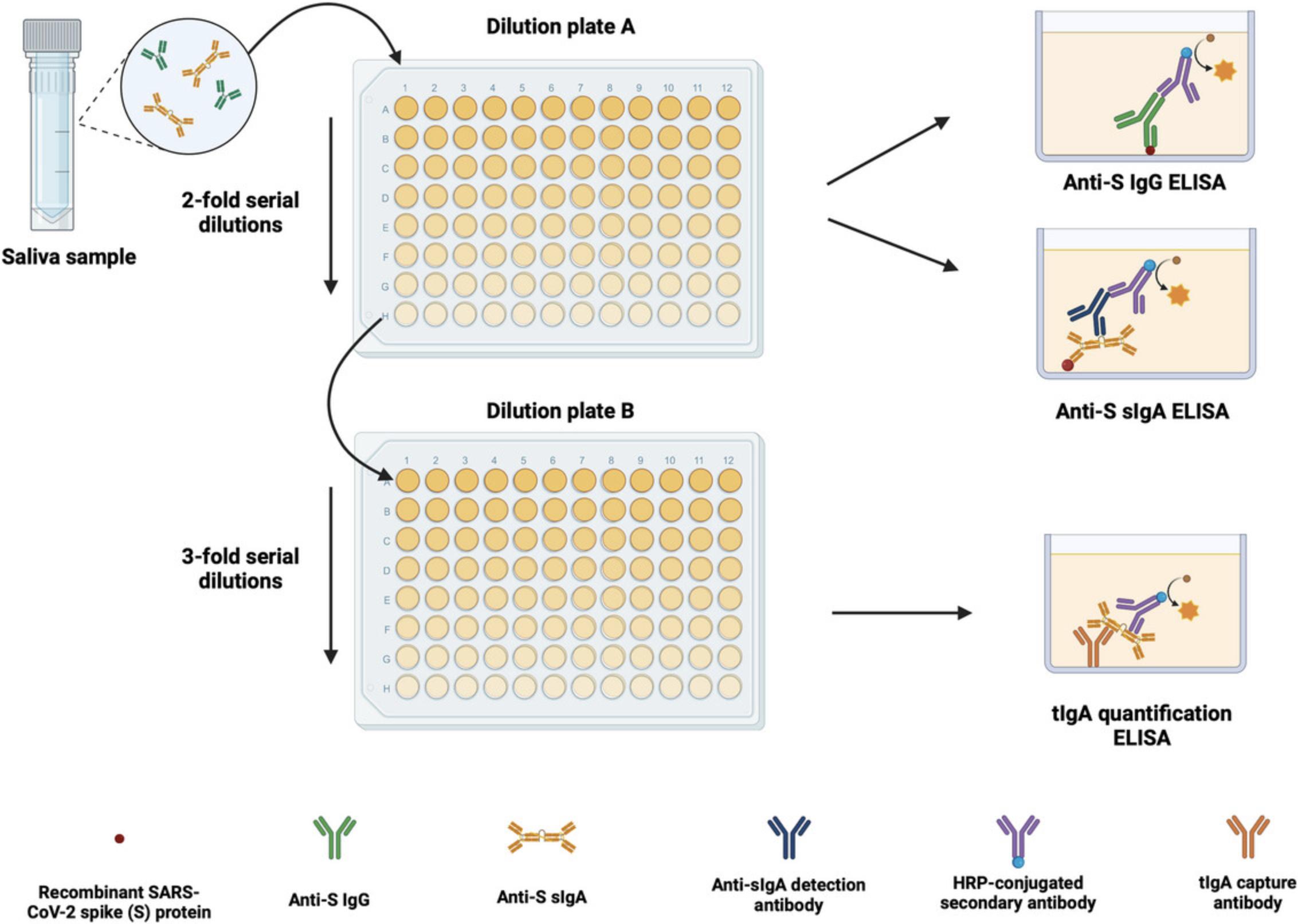
NOTE : Appropriate informed consent is necessary for obtaining and use of human study material.
Basic Protocol: INDIRECT ELISA TO MEASURE THE MUCOSAL ANTIBODY RESPONSE AGAINST SARS-CoV-2 SPIKE PROTEIN IN HUMAN SALIVA
Materials
-
Recombinant SARS-CoV-2 full-length spike protein (Stadlbauer et al., 2020; 10 µg per plate being coated for anti-S IgG and anti-S sIgA ELISA)
-
Phosphate-buffered saline (PBS), pH 7.4 (Gibco cat. no. 10010-023)
-
Anti-S IgG secondary antibody (capture antibody): Goat anti-Human IgG (H+L) Cross-Adsorbed Secondary Antibody, HRP conjugated (Invitrogen cat. no. 31412; see recipe)
-
Phosphate-buffered saline with 0.1% Tween 20 (PBS-T; see recipe)
-
Non-fat dry milk powder (AmericanBio cat. no. AB10109-01000)
-
Recombinant anti-S human IgG1 antibody clone CR3022 (ter Meulen et al., 2006; 480 ng per Dilution Plate A, as described in steps 11 and 14)
-
Recombinant anti-S human sIgA antibody clone CR3022 (antibody expression plasmids generously contributed by Dr. Tadaki Suzuki, National Institute of Infectious Diseases, Japan; 480 ng per Dilution Plate A, as described in steps 11 and 14)
-
Human IgA, Plasma (Sigma-Aldrich cat. no. 401098-2MG)
-
Saliva samples to be tested, frozen
-
tIgA capture antibody: Goat anti-Human IgA Antibody Affinity Purified (Bethyl Laboratories cat. no. A80-102A)
-
tIgA secondary antibody: Goat anti-Human IgA Antibody HRP conjugated (Bethyl Laboratories cat. no. A80-102P)
-
SIGMAFAST™ O -phenylenediamine dihydrochloride (OPD; Sigma-Aldrich cat. no. P9187)
-
3 M hydrochloric acid (HCl; Fisher Scientific cat. no. S25856)
-
sIgA detection antibody: Mouse anti-Human IgA, Secretory HP6141 (Millipore Sigma cat. no. 41142300UG)
-
Anti-S sIgA secondary antibody: Goat anti-Mouse IgG Fc Cross-Adsorbed Secondary Antibody, HRP conjugated (Invitrogen cat. no. 31439; see recipe)
-
Water for injection (WFI) for cell culture (Gibco cat. no. A1287301)
-
Class II biological safety cabinet (used for all steps involving human samples (saliva))
-
200-µl micropipet tips (USA Scientific cat. no. 1111-1700)
-
20-, 200-, and 1000-µl (P20, P200 and P1000) micropipets
-
15- and 50-ml sterile polypropylene conical tubes (Denville Scientific cat. nos. C1018P and C1060P)
-
5-, 10-, 25-, and 50-ml sterile serological pipets (Falcon cat. nos. 356543, 357551, 57535, and 356550)
-
ELISA plates: nonsterile flat-bottom immuno 96-Well Plates 4 HBX (Thermo Scientific cat. no. 3855)
-
Multichannel pipets capable of pipetting 50-250 µl
-
Sterile reservoirs (Fisher Scientific cat. no. 07-200-127)
-
Refrigerator, 4°C (±1°C)
-
Dilution plates: round-bottom 96-well cell culture plates with lids (Corning cat. no. 353227)
-
20- and 1000-µl barrier micropipet tips (Denville Scientific cat. nos. P1121 and P1126)
-
200-µl wide-bore micropipet tips (Thermo Scientific cat. no. 9405123)
-
1.5-ml microcentrifuge tubes (Denville cat. no. C2170)
-
Drummond Pipet-Aid
-
Vortex mixer
-
Kimberly-Clark Kimwipes (Kimberly-Clark Professional cat. no. 34721)
-
Timer
-
Biotek SynergyH1 microplate reader
-
Ultra-low-temperature freezer (–80°C)
-
Low-temperature freezer (–20°C)
-
Centrifuge for microcentrifuge tubes (mini centrifuge)
Day 1
Coating plates for anti-S IgG and sIgA ELISA
1.Thaw the required amount of recombinant spike protein and dilute to 2 µg/ml in 1× PBS.
2.Coat the 96-well flat-bottom microtiter plates (ELISA plates) with 50 µl/well of the diluted protein using a multichannel pipet. Tap the plates to ensure even coating at the bottom of every well.
3.Label half of the ELISA plates coated with spike protein for IgG ELISA and the other half for sIgA ELISA.
4.Cover the plates with a cover plate and incubate overnight at 4°C.
Coating plates for total IgA (tIgA) quantification ELISA
5.Dilute affinity-purified goat anti-human IgA antibody (capture antibody) to 5 µg/ml in 1× PBS.
6.Coat the 96-well flat-bottom microtiter plates (ELISA plates) with 50 µl/well of the diluted protein using a multichannel pipet. Tap the plates to ensure even coating at the bottom of wells.
7.Cover the plates with a cover plate and incubate overnight at 4°C.
Day 2
Blocking ELISA plates
8.Prepare blocking solution consisting of PBS-T + 5% (w/v) nonfat dry milk powder.
9.Discard the coating solution from the ELISA plates by inverting into an appropriate waste container and tap the plates dry on a Kimwipe or other absorbent towel. Add 200 µl blocking solution to each well. Cover with a cover plate and incubate for 1 hr at room temperature.
Preparing positive controls and standards
10.Prepare dilution buffer consisting of PBS-T + 2.5% (w/v) nonfat dry milk powder.
11.Prepare positive control for anti-spike IgG and sIgA ELISA: a combination of 2 µg/ml CR3022 IgG and 2 µg/ml CR3022 sIgA mAb is used as a positive control. To obtain this, combine CR3022 IgG and CR3022 sIgA mAb, each at a concentration of 2 µg/ml, in dilution buffer.
12.Prepare standard for total IgA quantification: Dilute human plasma IgA to 2 µg/ml in dilution buffer.
Diluting saliva samples
13.For each sample set, prepare two round-bottom pre-dilution plates, plate A and plate B. Diluted samples in plate A will be subjected to anti-spike sIgA and IgG ELISA whereas diluted samples in plate B will be subjected to total IgA quantification ELISA.
14.For plates A, add 120 µl dilution buffer to all wells. Add an additional 120 µl to wells A1 and A12.Add 120 µl of the positive control prepared in step 11 to wells A10 and A11.
15.Thaw saliva samples and vortex prior to use. Add 120 µl of saliva samples to wells A2-A9.Using a multichannel pipet, mix row A by pipetting up and down. Using fresh tips, transfer 120 µl from wells in row A to the respective wells in row B. Continue with serial dilution until row H. Change pipette tips at every step.
16.For plates B, add 80 µl of dilution buffer to all wells in row B-H. Add an additional 120 µl to wells A1 and A12.Add 120 µl of the standard prepared in step 12 to wells A10 and A11.
17.Using fresh micropipet tips, transfer 80 µl from wells H2-H9 of plate A to wells A2-A9 of corresponding plate B. Change tips and transfer 40 µl from wells in row A to respective wells in row B. Continue serial dilution until row H and discard final 40 µl from row H. For reference, see dilution plate layout in Figure 2.
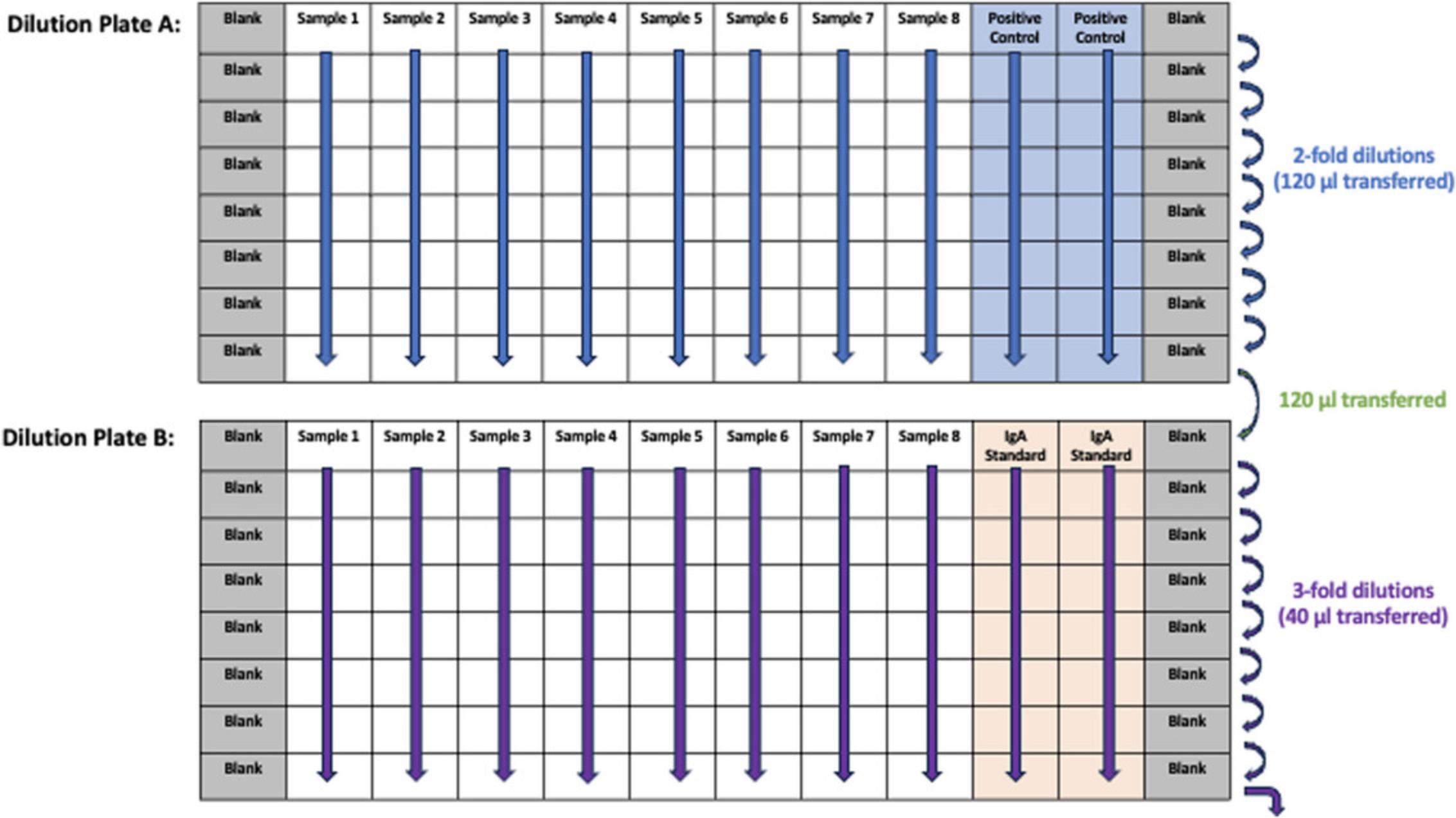
Adding diluted samples to ELISA plates
18.Remove blocking solution from ELISA plates. Tap the plates dry on a Kimwipe or other absorbent material.
19.Transfer 50 µl of diluted sample from row A of the dilution plate A to row A of the corresponding IgG ELISA plate. Using the same tips, transfer 50 µl of diluted sample from row A of the dilution plate A to row A of the corresponding sIgA ELISA plate. Change tips and continue to transfer the second row of the dilution plate to the ELISA plates in the same manner.
20.Transfer 50 µl of diluted sample from row A of the dilution plate B to row A of corresponding total IgA quantification ELISA plate. Continue until row H and change tips between rows each time.
21.Incubate the plates labeled for sIgA overnight (18-24 hr) at 4°C and then proceed to step 31 with these plates. Incubate the plates labeled for IgG and tIgA for 2 hr at room temperature and continue with step 22.
Adding detection antibody to anti-S IgG and tIgA ELISA plates
22.Wash anti-S IgG and tIgA ELISA plates three times with 200 µl/well of PBS-T.
23.Prepare secondary antibody for anti-S IgG ELISA: Prepare a 1:2500 dilution of anti-S IgG secondary antibody (Goat anti Human IgG (H+L) Cross-Adsorbed Secondary Antibody, HRP conjugated) in dilution buffer. Add 50 µl to each well of the anti-S IgG ELISA plates.
24.Prepare secondary antibody for tIgA quantification ELISA: Prepare a 1:1000 dilution of Goat anti-Human IgA, Antibody, HRP conjugated, in dilution buffer. Add 50 µl to each well of the IgG ELISA plates.
25.Incubate anti-S IgG and tIgA ELISA plates for 1 hr at room temperature.
Developing anti-S IgG and tIgA ELISA plates
26.Wash anti-S IgG and tIgA ELISA plates three times with 200 µl/well of PBS-T.
27.Prepare 10 ml of OPD solution for each plate. One set of tablets (one urea hydrogen peroxide tablet and one OPD tablet) can be dissolved in 20 ml of water to prepare 20 ml of OPD solution.
28.Add 100 µl of OPD solution to each well of the anti-S IgG and tIgA ELISA plates. Incubate for 10 min at room temperature.
29.At the end of the incubation time, stop the reaction by adding 50 µl of 3 M HCl to each well.
30.Immediately read ELISA plates at an absorbance of 490 nm using an automated plate reader and record data.
Day 3
Adding detection antibody to anti-S sIgA ELISA plates
31.At the end of overnight incubation described in step 21, wash anti-S sIgA ELISA plates three times with 200 µl/well of PBS-T.
32.Prepare detection antibody for anti-S sIgA ELISA: Prepare 5 µg/ml of Mouse anti-Human IgA, Secretory (H6141), in dilution buffer. Add 50 µl to each well of the anti-S sIgA ELISA plates. Incubate 2 hr at room temperature.
33.Wash anti-S sIgA ELISA plates three times with 200 µl/well of PBS-T.
34.Prepare secondary antibody for anti-S sIgA ELISA: Prepare 1:1000 dilution of anti-S sIgA secondary antibody (Goat anti Human IgG Fc Cross-Adsorbed Secondary Antibody, HRP conjugated) in dilution buffer. Add 50 µl to each well of the anti-S sIgA ELISA plates. Incubate 1 hr at room temperature.
Developing anti-S sIgA ELISA plates
35.Wash anti-S sIgA ELISA plates three times with 200 µl/well of PBS-T.
36.Prepare 10 ml of OPD solution for each plate. One set of tablets (one urea hydrogen peroxide tablet and one OPD tablet) can be dissolved in 20 ml of water to prepare 20 ml of OPD solution.
37.Add 100 µl of OPD solution to each well of the anti-S sIgA ELISA plates. Incubate for 10 min at room temperature.
38.At the end of the incubation time, stop the reaction by adding 50 µl of 3 M HCl to each well.
39.Immediately after adding HCl, read ELISA plates at an absorbance of 490 nm using an automated plate reader and record data.
Data analysis
40.For each IgG and sIgA ELISA plate, determine the baseline by taking the average of the absorbance values recorded for the blank wells (columns 1 and 12) and adding three times the standard deviation. Use this value to calculate the area under the curve (AUC) value for each sample on the plate using GraphPad Prism or similar data analysis software.
41.For each total IgA quantification plate, open an XY table I in GraphPad Prism. Select “Numbers” for X and “Enter and plot a single Y value for each point” for Y. Add standard IgA concentration (2000-0.914) to X. Add the optical density (OD) values for IgA standard (column 10 and 11) to columns A:Y1 and A:Y2 of the table in rows 1-8.Paste the OD values for the samples under column A:Y1 (row 9-16 for sample 1, 17-24 for sample 2, and so on). Interpolate the standard curve for the plate using the “Asymmetric Sigmoidal, 5PL X is concentration” function. Record the interpolated values in a separate sheet.
42.For each sample on the plate, select the lowest dilution at which the OD value is within the range of 0.5-1.5.Calculate the IgA concentration in the sample by multiplying the interpolated concentration at the selected dilution by the dilution factor of the sample. Divide the result by 1000. The result is the IgA concentration within the sample in µg/µl units.
43.To calculate the adjusted AUC for the sIgA plates, divide the AUC value obtained for each sample in step 11a by the concentration of IgA in the corresponding sample obtained in step 11c, and multiply this value by 100. The result is the “Adjusted AUC value” for sIgA measured in the sample.
44.The result reported for anti-S IgG ELISA is the AUC value, while the result for anti-S sIgA ELISA is the adjusted AUC value.
REAGENTS AND BUFFERS
Anti-S IgG secondary antibody, 0.8 mg/ml
Add 750 µl water for injection (WFI; Gibco cat. no. A1287301) and 750 µl glycerol (Fisher Scientific cat. no. BP2291) to 1 vial of lyophilized Goat anti-Human IgG (H+L) Cross-Adsorbed Secondary Antibody, HRP conjugated (Invitrogen cat. no. 31412). Mix well by pipetting up and down and make 50-µl aliquots. Store for up to 2 years at −20°C.
Anti-S sIgA secondary antibody, 0.8 mg/ml
Add 750 µl water for injection water for injection (WFI; Gibco cat. no. A1287301) and 750 µl glycerol (Fisher Scientific cat. no. BP2291) to 1 vial of lyophilized Goat anti-Human IgG Fc Cross-Adsorbed Secondary Antibody, HRP conjugated (Invitrogen cat. no. 31439). Mix well by pipetting up and down and make 50-µl aliquots. Store for up to 2 years at −20°C.
Phosphate-buffered saline with 0.1% (v/v) Tween 20 (PBS-T; 50 liters)
Combine 5 liters of 10× PBS (Corning™ cat. no. 46013CM) and 50 ml of Tween 20 (Fisher Bioreagents cat. no. BP337-500), add distilled water to 45 liters total, and mix well. Store for up to 4 months at room temperature.
COMMENTARY
Background Information
This protocol has been developed based on previously established ELISA protocols for measuring IgG antibodies in serum (Stadlbauer et al., 2020). However, due to the nature of saliva samples (e.g., the relatively high background signals when used in ELISA), some parts of the protocol have been optimized for the measurement of anti-S IgG and sIgA levels within saliva samples. One characteristic of this protocol is that the result of anti-S specific sIgA ELISA is normalized based on the total IgA concentration of each saliva sample. Various protocol parameters have been tested to determine the optimal conditions.
For the sIgA detection ELISA, we determined the best incubation time for coating with antigen to be 18-24 hr, as the coating efficiency of the recombinant antigen is not impacted in this window. sIgA signals within saliva samples are typically quite low. Increasing the sample incubation time to 24 hr improves the detection of sIgA within the sample and helps mitigate the issue of low signals. Sample incubation times within 18-24 hr typically lead to improved sIgA detection. Different concentrations of the sIgA detection antibody and the secondary horseradish peroxidase (HRP)-conjugated antibody were tested to determine the highest signal-to-noise ratio value.
For the total IgA quantification ELISA, different coating concentrations and incubation times for the capture antibody were tested. The highest signal-to-noise ratio was obtained when the plates were coated with 50 µl/well of 5 µg/ml capture antibody, and this does not vary significantly when the plates are incubated at 4°C for 18 or 24 hr. The optimal dilution of the detection antibody was determined to be 1:1000.
The protocol described here was developed for measuring anti-spike antibodies in saliva, but other respiratory fluids, such as nasal secretions or bronchoalveolar fluid or washes, could potentially be used too. Furthermore, the setup could potentially also be used to measure antibodies to other virus glycoproteins, such as influenza virus hemagglutinin or neuraminidase. However, these changes may require re-optimization of the protocol.
Critical Parameters and Troubleshooting
Saliva samples are often viscous and can cause errors in pipetting that affect the dilutions. We recommend vortexing the samples before use to make them more homogenous. Use of wide-bore tips is also helpful for accurate pipetting. Thawed saliva samples can be stored at 4°C for up to 24 hr; however, −80°C is recommended for long-term storage. Samples can be refrozen and stored at −80°C, but repeat freeze-thaw should be avoided. The quality of the antigen used to coat the IgG and sIgA ELISA plates can also affect the outcome of the assay. Each lot of antigen should be checked for degradation using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) before use. Some common problems encountered with this protocol, the possible causes, and suggested solutions are listed in the Table 1.
| Problem | Possible cause | Solution |
|---|---|---|
| High background | Improper washing of plates | Perform wash steps by hand with utmost care instead of using automated plate washer |
| Exceeding incubation time with secondary antibody | Adhere to incubation times closely: do not exceed recommended time by >5 min | |
| Low reactivity for positive control | Use of old OPD solution | OPD solution must be prepared immediately before use |
| Multiple peaks in AUC analysis | Error in serial dilution |
Up to two values that are out of range can be excluded from AUC calculation If more values are found to be out of range, perform serial dilutions carefully, changing tips between each dilution step |
Understanding Results
The readout for the ELISA for anti-S IgG and sIgA is the absorbance measured at 490 nm. The curve generated by the absorbance values should follow a regular dilution pattern. The area under the curve (AUC) generated by the values measured across sample dilutions corresponds to the amount of IgG or sIgA antibodies present within the sample. In the AUC analysis, a single peak should be detected. sIgA measurements are adjusted according to the concentration of total amount of IgA present within the sample to eliminate the bias introduced by variation in the amount of IgA due to other factors. The absorbance values for human IgA standard obtained for tIgA ELISA are used to generate a standard curve. The concentrations of IgA for the samples within every plate are extrapolated based on the standard curve generated for that plate. The standard curve should be sigmoidal and follow the dilution pattern. Representative examples of samples evaluated for anti-S IgG and sIgA, as well as a standard curve interpolated from the values obtained for the IgA standard used in tIgA ELISA, are shown in Figure 3 for reference.
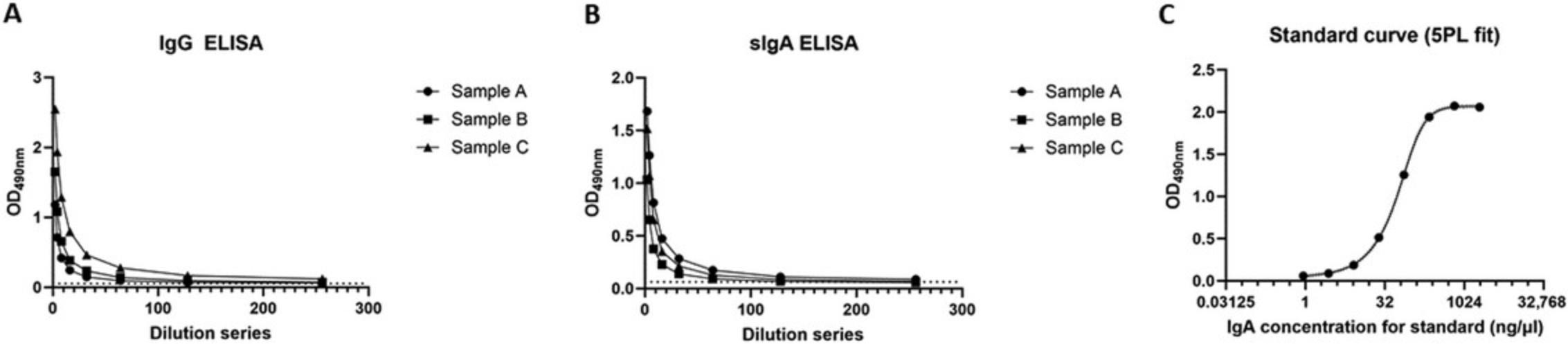
Time Considerations
The protocol takes 3 days, with coating of antigen for spike-specific IgG and sIgA ELISA, and capture antibody for tIgA quantification ELISA on day 1. The IgG and total IgA quantification ELISA are completed on day 2, along with addition of samples to the sIgA plates followed by overnight incubation. The sIgA ELISA is then continued and completed on day 3.
Acknowledgments
The authors thank Vivian A. Simon for generously supplying saliva samples for the development of this assay. The assay development and immune analysis work was funded by the Serological Sciences Network (SeroNet) in part with U.S. Federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. 75N91019D00024, Task Order No. 75N91021F00001. Work on mucosal antibody responses to SARS-CoV-2 in the Krammer laboratory is also supported by the NIAID Centers of Excellence for Influenza Research and Response (CEIRR, contract no. 75N93021C00014) and by the Collaborative Influenza Vaccine Innovation Centers (CIVICs contract no. 75N93019C00051).
Author Contributions
Disha Bhavsar : Conceptualization; data curation; formal analysis; investigation; methodology; validation; visualization; writing—original draft; writing—review and editing. Kaori Sano : Conceptualization; data curation; formal analysis; investigation; methodology; writing—original draft; writing—review and editing. Gagandeep Singh : Data curation; investigation; methodology; writing—original draft; writing—review and editing. Florian Krammer : Conceptualization; funding acquisition; project administration; supervision; writing—original draft; writing—review and editing.
Conflict of Interest
The Icahn School of Medicine at Mount Sinai has filed patent applications relating to SARS-CoV-2 serological assays, NDV-based SARS-CoV-2 vaccines, influenza virus vaccines, and influenza virus therapeutics that list Florian Krammer as co-inventor, several of which are licensed to commercial entities. Mount Sinai has spun out a company, Kantaro, to market serological tests for SARS-CoV-2 and another company, Castlevax, to develop SARS-CoV-2 vaccines. Florian Krammer is co-founder and scientific advisory board member of Castlevax. Florian Krammer has consulted for Merck, Curevac, Seqirus and Pfizer and is currently consulting for 3rd Rock Ventures, GSK, Gritstone and Avimex. The Krammer laboratory is also collaborating with Dynavax on influenza vaccine development.
Open Research
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Literature Cited
- Bhavsar, D., Singh, G., Sano, K., Gleason, C., Srivastava, K., PARIS Study Group, Carreno, J. M., Simon, V., & Krammer, F. (2023). Mucosal antibody responses to SARS-CoV-2 booster vaccination and breakthrough infection. MBio , 14(6), e0228023. https://doi.org/10.1128/mbio.02280-23
- Brandtzaeg, P. (2007). Induction of secretory immunity and memory at mucosal surfaces. Vaccine , 25(30), 5467–5484. https://doi.org/10.1016/j.vaccine.2006.12.001
- Havervall, S., Marking, U., Svensson, J., Greilert-Norin, N., Bacchus, P., Nilsson, P., Hober, S., Gordon, M., Blom, K., Klingström, J., Åberg, M., Smed-Sörensen, A., & Thålin, C. (2022). Anti-spike mucosal IgA protection against SARS-CoV-2 omicron infection. New England Journal of Medicine , 387(14), 1333–1336. https://doi.org/10.1056/NEJMc2209651
- Marking, U., Bladh, O., Havervall, S., Svensson, J., Greilert-Norin, N., Aguilera, K., Kihlgren, M., Salomonsson, A. C., Månsson, M., Gallini, R., Kriegholm, C., Bacchus, P., Hober, S., Gordon, M., Blom, K., Smed-Sörensen, A., Åberg, M., Klingström, J., & Thålin, C. (2023). 7-month duration of SARS-CoV-2 mucosal immunoglobulin-A responses and protection. The Lancet Infectious Diseases , 23(2), 150–152. https://doi.org/10.1016/s1473-3099(22)00834-9
- Martines, R. B., Ritter, J. M., Matkovic, E., Gary, J., Bollweg, B. C., Bullock, H., Goldsmith, C. S., Silva-Flannery, L., Seixas, J. N., Reagan-Steiner, S., Uyeki, T., Denison, A., Bhatnagar, J., Shieh, W. J., Zaki, S. R., & COVID-19 Pathology Working Group. (2020). Pathology and Pathogenesis of SARS-CoV-2 Associated with fatal coronavirus disease, United States. Emerging Infectious Diseases , 26(9), 2005–2015. https://doi.org/10.3201/eid2609.202095
- Sano, K., Bhavsar, D., Singh, G., Floda, D., Srivastava, K., Gleason, C., PARIS Study Group, Carreño, J. M., Simon, V., & Krammer, F. (2022). SARS-CoV-2 vaccination induces mucosal antibody responses in previously infected individuals. Nature Communications , 13(1), 5135. https://doi.org/10.1038/s41467-022-32389-8
- Sheikh-Mohamed, S., Isho, B., Chao, G. Y. C., Zuo, M., Cohen, C., Lustig, Y., Nahass, G. R., Salomon-Shulman, R. E., Blacker, G., Fazel-Zarandi, M., Rathod, B., Colwill, K., Jamal, A., Li, Z., de Launay, K. Q., Takaoka, A., Garnham-Takaoka, J., Patel, A., Fahim, C., … Gommerman, J. L. (2022). Systemic and mucosal IgA responses are variably induced in response to SARS-CoV-2 mRNA vaccination and are associated with protection against subsequent infection. Mucosal Immunology , 15(5), 799–808. https://doi.org/10.1038/s41385-022-00511-0
- Stadlbauer, D., Amanat, F., Chromikova, V., Jiang, K., Strohmeier, S., Arunkumar, G. A., Tan, J., Bhavsar, D., Capuano, C., Kirkpatrick, E., Meade, P., Brito, R. N., Teo, C., McMahon, M., Simon, V., & Krammer, F. (2020). SARS-CoV-2 seroconversion in humans: A detailed protocol for a serological assay, antigen production, and test setup. Current Protocols in Microbiology , 57(1), e100. https://doi.org/10.1002/cpmc.100
- Sun, W., Liu, Y., Amanat, F., González-Domínguez, I., McCroskery, S., Slamanig, S., Coughlan, L., Rosado, V., Lemus, N., Jangra, S., Rathnasinghe, R., Schotsaert, M., Martinez, J. L., Sano, K., Mena, I., Innis, B. L., Wirachwong, P., Thai, D. H., Oliveira, R. D. N., … Palese, P. (2021). A Newcastle disease virus expressing a stabilized spike protein of SARS-CoV-2 induces protective immune responses. Nature Communications , 12(1), 6197. https://doi.org/10.1038/s41467-021-26499-y
- Suzuki, T., Kawaguchi, A., Ainai, A., Tamura, S., Ito, R., Multihartina, P., Setiawaty, V., Pangesti, K. N., Odagiri, T., Tashiro, M., & Hasegawa, H. (2015). Relationship of the quaternary structure of human secretory IgA to neutralization of influenza virus. Proceedings of the National Academy of Sciences of the United States of America , 112(25), 7809–7814. https://doi.org/10.1073/pnas.1503885112
- ter Meulen, J., van den Brink, E. N., Poon, L. L., Marissen, W. E., Leung, C. S., Cox, F., Cheung, C. Y., Bakker, A. Q., Bogaards, J. A., van Deventer, E., Preiser, W., Doerr, H. W., Chow, V. T., de Kruif, J., Peiris, J. S., & Goudsmit, J. (2006). Human monoclonal antibody combination against SARS coronavirus: Synergy and coverage of escape mutants. PLoS Medicine , 3(7), e237. https://doi.org/10.1371/journal.pmed.0030237
- Wu, F., Zhao, S., Yu, B., Chen, Y. M., Wang, W., Song, Z. G., Hu, Y., Tao, Z. W., Tian, J. H., Pei, Y. Y., Yuan, M. L., Zhang, Y. L., Dai, F. H., Liu, Y., Wang, Q. M., Zheng, J. J., Xu, L., Holmes, E. C., & Zhang, Y. Z. (2020). A new coronavirus associated with human respiratory disease in China. Nature , 579(7798), 265–269. https://doi.org/10.1038/s41586-020-2008-3
- Zhu, N., Zhang, D., Wang, W., Li, X., Yang, B., Song, J., Zhao, X., Huang, B., Shi, W., Lu, R., Niu, P., Zhan, F., Ma, X., Wang, D., Xu, W., Wu, G., Gao, G. F., Tan, W., & China Novel Coronavirus Investigating and Research Team. (2020). A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine , 382(8), 727–733. https://doi.org/10.1056/NEJMoa2001017

